Ovarian cancer (OC) is the most lethal gynaecological cancer. In 2010, approximately 21,880 new cases and 13,850 deaths occurred in the US,1 while in the EU in 2004 the incidence of newly diagnosed cases was 42,700 with a mortality of 12/100,000 women/year.2 The current treatment of OC involves aggressive cytoreductive surgery followed by platinum- and taxane-based chemotherapy, which produces an objective response rate in approximately 65–80% of patients.3
Ovarian cancer (OC) is the most lethal gynaecological cancer. In 2010, approximately 21,880 new cases and 13,850 deaths occurred in the US,1 while in the EU in 2004 the incidence of newly diagnosed cases was 42,700 with a mortality of 12/100,000 women/year.2 The current treatment of OC involves aggressive cytoreductive surgery followed by platinum- and taxane-based chemotherapy, which produces an objective response rate in approximately 65–80% of patients.3
Nevertheless, despite surgery and first-line chemotherapy, between 60 % and 70 % of OC patients will recur and their prognosis and quality of life remain poor.4 Platinum-based combination therapies represent the standard care for patients recurring more than six months after initial therapy;5 combination treatment with paclitaxel and gemcitabine have been reported to increase outcome in terms of platinum monotherapy in recurrent platinum-sensitive patients.6 Recently, the Aarboplatin and Pegylated Liposomal Doxorubicin [PLD] versus Carboplatin and Paclitaxel in Relapsed Platinum-sensitive Ovarian cancer (CALYPSO) trial reported a better therapeutic index for the combination of carboplatin-PLD in comparison with carboplatinpaclitaxel in recurrent, platinum-sensitive OC thus representing, for most clinicians, the preferred option in this setting.7
The response to platinum retreatment is related to the length of prior response to platinum.8,9It has been postulated that patients with partially platinum-sensitive recurrent OC (platinum-free interval [PFI] between six to 12 months) may benefit from a strategy of artificially extending the PFI by using a non-platinum agent followed by platinum at subsequent relapse.10 In this view, after the publication of An Efficacy and Safety Study for Yondelis (Trabectedin) in Patients With Advanced Relapsed Ovarian Cancer (OVA-301) results,11 the combination of trabectedin-PLD represent, for several clinicians, an alternative option to platinum-based regimens in this setting.
In general, platinum-resistant patients (who are patients recurring less than six months from last platinum treatment) are treated with sequential single non-platinum agents rather than with combination therapy4and, currently, the drugs approved in this subset of patients are paclitaxel, PLD, etoposide and topotecan with a short-lasting response rate in 10–25% of patients and a general dismal prognosis.8,9 Therefore, novel treatment strategies are required to improve outcomes in women with recurrent OC, particularly in resistant disease.
Human NGR-Tumour Necrosis Factor
Tumour necrosis factor (TNF) was first identified by Carswell et al. in the serum of Bacillus Calmette-Guerin-sensitised animals treated with an endotoxin causing the release of a factor able to induce haemorrhagic necrosis of murine tumours.12
In an in vitro experiment TNF has been shown to have cytostatic and cytotoxic effects against a wide range of human tumour cells and human tumour xenografts in nude mice.13,14
In addition to the well-known cytostatic/cytotoxic properties, TNF has a broad spectrum of immunomodulatory activities.15TNF enhances the expression of class I major histocompatibility antigens on human endothelial cells, dermal fibroblasts and human tumour cell lines16,17 and the expression of Class II major histocompatibility antigens on human T cells and tumour cells.18 TNF has also demonstrated multiple actions on natural killer cells, macrophage cells and granulocyte function.19,20,21 Moreover, evidence suggests that the anti-tumour activity of TNF depends on indirect mechanisms associated with selective obstruction and damage of tumour-associated blood vessels and on activation of immune mechanisms rather than a direct toxic effect on tumour cells.23
Although numerous preclinical studies have demonstrated that TNF has notable anti-tumour activity12,14,22,24early trials in humans showed that its clinical use was limited by severe systemic toxicity.13,15,25–27 Despite this, loco-regional therapy with TNF high doses in combination with chemotherapy led to elevated response rates in patients with melanoma and sarcoma of the extremities27–31 and regression of bulky hepatic cancers confined to the liver30 and peritoneal carcinomas.31
In vivo screening of peptide-phage libraries has proven to be a powerful tool for the discovery of ligands that selectively home to tumour vessels.32,33 Among the targeting probes, a peptide containing the NGR motif has been coupled to different anticancer compounds, such as doxorubicin, and TNF, thus enabling targeted delivery of these drugs to tumour vessels.23,33,34 Based on those observations, the coupling of murine TNF (mTNF) with peptide CNGRC, an aminopeptidase ligand (CD13 antigen), capable of binding to tumour blood vessels, demonstrated therapeutic properties superior to those of mTNF in animal models.23A modified human TNF (hTNF) containing the CNGRC peptide sequence was subsequently prepared using recombinant DNA techniques.23 The resulting NGR-hTNF will enable targeted delivery of TNF to tumour vessels in humans.
The anticancer activity of NGR-hTNF was investigated in a preclinical series of experiments aimed at demonstrating the ability of the cytokine in inducing regression of neoplastic lesions both as single agent and in combination with chemotherapeutic drugs.
Preclinical anti-tumour activity studies showed that murine NGRTNF (NGR-mTNF) is almost 12 to 15 times more effective than mTNF in suppressing growth of RMA-T and RMA lymphoma and B16F1 melanoma in mice with similar toxicity.23A similar potency differential was observed between NGR-hTNF and hTNF against RMA-T tumours in mice, thus a similar effect would be expected for NGR-hTNF in man offering a better risk–benefit in cancer treatment.
The pharmacokinetic properties were investigated for both NGR-TNF and TNF and the dose–response curve, obtained for NGR-TNF was compared with similar amounts of TNF (at doses of 0.01, 1, 10, 100, 1,000 and 10,000 ng). In this experimental model, doses included between 0.01 to 1 ng/mouse of NGR-hTNF (considered low doses) were sufficient to induce maximal effect, defined as tumour shrinkage; by contrast, in this experimental model, TNF was active only at high doses (1,000 and 10,000 ng/mouse).32
When administered as a single agent, NGR-TNF showed activity in inducing tumour regression either at low doses (0.01–0.1 ng) or at high doses (1,000–10,000 ng), suggesting a bell-shaped dose–response curve. Moreover, low doses of NGR-TNF (0.1 ng) were demonstrated to enhance the anti-tumour activity of chemotherapy, such as doxorubicin,32 cisplatin and melphalan, which indicated a synergistic effect with no evidence of increased toxicity. In particular, NGR-mTNF improved doxorubicin penetration in tumours, as the percentage of cancer cells that can be reached by doxorubicin and the drug intracellular amount suggests that NGR-mTNF improves cytotoxic drug penetration in tumours.32 The delivery of minute amounts of TNF to tumour vessels represents a novel approach for avoiding negative feedback mechanisms and preserving its ability to alter drug-penetration barriers. Vascular targeting could represent a novel strategy for increasing the therapeutic index of chemotherapeutic drugs.
NGR-Human Tumour Necrosis Factor– Doxorubicin Combination
Anthracyclines are among the most used chemotherapy agents in several solid and haematological tumours: anthracyclines exert claimed anti-tumour activity in OC; nevertheless, the effect on survival was demonstrated only in isolated clinical trials.35 In fact, in most studies the addition of doxorubicin had no effect on survival – only affecting the safety profile of the treatment especially in terms of myelotoxicity – and some authors reported that this could be related to the poor penetration of doxorubicin into cancer cells.36
The relatively poor penetration of doxorubicin in cancer cells is probably related to the structural and functional abnormalities typical of tumour disorganised vasculature and to the complete lack of lymphatics responsible of heterogeneous tumour perfusion, abnormal vascular permeability and increased interstitial pressure. These critical barriers may limit the penetration of drugs into cancer cells distant from tumour vessels and, consequently, the effectiveness of chemotherapy.36 Therapeutic strategies that enhance drug penetration have therefore considerable potential to increase cell killing and, consequently, tumour sensitivity.
Preclinical data demonstrated that when administered in combination to doxorubicin, NGR-mTNF exerts a synergic cytotoxic activity probably enhancing tumour cell penetration of the drug without changing the pharmacokinetics and/or increasing the toxicity profile of doxorubicin. In mouse models bearing WEHI-164 fibrosarcoma cells treated with 0.1 ng/ mouse of NGR-mTNF followed by the administration of doxorubicin, the maximal synergism between drugs was observed when doxorubicin was administered two hours after the infusion of NGR-mTNF.37
A preclinical study was performed in cynomolgus monkey in order to define the safety profile of the combination of NGR-hTNF plus doxorubicin. The NGR-hTNF administered intravenously to cynomolgus monkeys at the doses of 0, 50 and 1,000 ng/Kg in combination with doxorubicin at 4 mg/kg every three weeks was well tolerated: toxic effects were observed in the haemolymphopoietic system, at the injection site and in lachrymal glands, and were considered related to doxorubicin. No findings attributable to NGR-hTNF or exacerbation of effects induced by doxorubicin were noted.37
In order to assess the safety profile of doxorubicin and NGR-hTNF when administered sequentially in humans, a phase Ib clinical trial (NGR003) was carried out and 15 patients were treated with NGR-hTNF at lowdose escalation between 0.2 and 1.6 μg/m2 along with doxorubicin at a fixed dose of 75 mg/m2. The most frequently NGR-hTNF-related adverse events (AEs), reported in at least 10 % of patients, were rigors, nausea, fatigue, hypertension, vomiting and pyrexia. As far as anti-tumour activity is concerned, stabilisation of disease occurred in 10/15 patients (66 %) and partial long-lasting (23 weeks) responses were achieved in two patients (13 %). Of note, the achievement of the disease control rates (stabilisations of disease [SD] and partial response [PR]) in 12/15 patients – nine of whom previously treated with anthracyclines and five with resistant disease. Overall, the combination treatment showed a favourable safety profile and encouraging cytotoxic activity suggesting that NGR-hTNF was suitable to be further explored in combination with doxorubicin within a phase II programme.
At our institution, a phase II prospective study was carried out in a population of recurrent, resistant and partially platinum-sensitive OC with progressive disease after at least one previous platinumbased chemotherapy line.38 Thirty-seven patients with radiologically documented progressive disease were enrolled and 35 were evaluable for response: eight partial responses (23 %; 95 % confidence interval [CI] 11–40 %) and 15 stabilisations of diseases (43 %; 95 % CI 26–61 %) were registered; median progression-free survival (PFS) was 5.0 months for the whole study population and, in particular, 8.2 months for patients who achieved a PR and 4.9 months for patients experiencing SD. Among 23 patients evaluable for Gynecologic Cancer InterGroup (GCIG) CA-125 criteria, nine responses (39 %) were recorded.
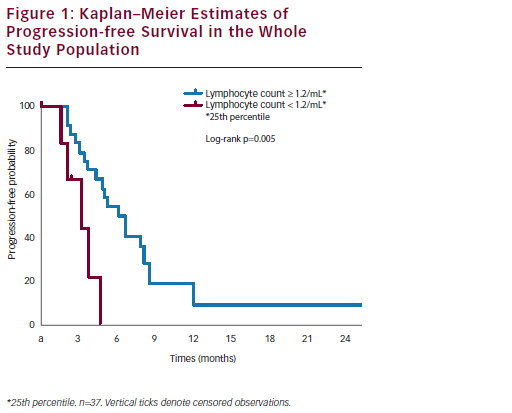
After a median follow up of 14.6 months, the one-year survival rate was 65%; median survival was 24.0 months in patients with PR or SD and 4.9 months in patients with progression of disease (p<0.0001) (see Figure 1).
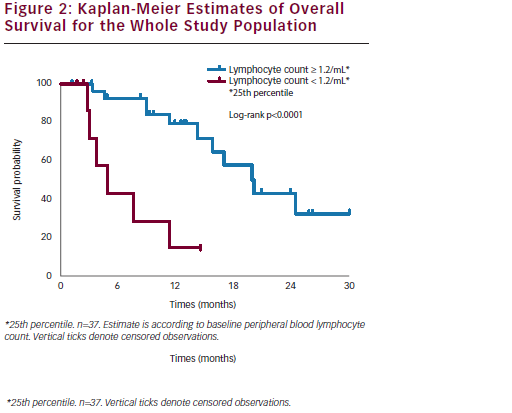
Interestingly, a correlation was documented between basal peripheral blood lymphocyte count (PBLC) and outcome: in the subset of refractory/resistant patients with PBLC higher or lower than 1.2/mL (i.e. the 25th percentile distribution value) a median PFS of 4.9 and 2.6 months (p=0.02) and a median OS of 15.8 and 4.3 months (p=0.0001), respectively, were registered (see Figure 2). In univariate Cox analyses, both longer PFI (p=0.03) and higher baseline PBLC (p=0.01) were significantly associated with better PFS, and only overall survival correlated with PBLC (p=0.001).
Chemotherapy treatment appeared well tolerated: most common grade 3 non-haematological AE were asthenia (3 % of patients); two patients (5 %) reported grade 1 cardiac AE (one tachycardia and one diastolic dysfunction). Only 9 % of all recorded AEs were related to NGR-hTNF infusion and were generally represented by short-lived, infusion-related, grade 1 to 2 chills (65 % of patients). In terms of haematological toxicity, grade 3 and 4 neutropoenia were registered in seven (19 %) and 16 patients (43 %), respectively, with grade 3 and 4 anaemia in nine (24 %) and one patient (3 %), respectively. Only one episode of neutropenic fever was reported (3 %). All cycles were given at the planned dose, except for eight patients (22 %) in which a doxorubicin dose reduction was required.
We concluded that NGR-hTNF-doxorubicin combination retains interesting clinical activity and a secure toxicity profile in recurrent OC. The evidence of disease control achieved in more than half of patients and maintained for a median of more than six months is of note particularly in the resistant recurrent setting; moreover, in our study a low lymphocyte count at baseline seems to predict early progression and short survival, whereas a normal lymphocyte count seems to correlate with prolonged clinical benefit. These clinical observations are consistent with preclinical evidence of a synergism between NGRhTNF and doxorubicin, reported only in immunocompetent mice, being lacking in nude mice depleted of functionally mature T lymphocytes.39 From this viewpoint, the baseline PBLC could be an easily accessible and reproducible predictive biomarker of outcome, which might be exploited for selecting refractory/resistant OC patients who would mostly benefit from the combination of NGR-hTNF and doxorubicin (see Figure 2).
Toxicity profiles of the two drugs apparently did not overlap; most common AEs are those expected when each agent is given alone, consisting of neutropoenia for doxorubicin and chills for NGR-hTNF. More interestingly, cardiovascular toxicities previously reported for vascular targeting agents40 did not appear in our study and, even more importantly, despite half of patients completing six or more cycles of doxorubicin, there was no exacerbation of the well-known cardiac toxicity profile of doxorubicin.
Given the outstanding use of PLD in OC and the synergism documented in preclinical models between low-dose TNF and liposomal formulation of doxorubicin,41 a randomised phase II trial (NCT01358071) trial testing PLD with or without NGR-hTNF in recurrent, platinum-refractory/resistant OC is ongoing in several Italian institutions.