Mature cystic teratomas (also called dermoid cysts) account for about 30–45 % of all ovarian neoplasms and around 60 % of all benign tumours arising in the ovary.1 Malignant transformation of the various mature tissue components of a dermoid cyst is rare and the reported incidence is 0.17–1.4 %.2,3 Squamous cell carcinoma (SCC), arising from the ectodermal component, is the commonest form of malignant transformation accounting for >80 % of cases, followed by adenocarcinomas and carcinoid tumours.4
Mature cystic teratomas (also called dermoid cysts) account for about 30–45 % of all ovarian neoplasms and around 60 % of all benign tumours arising in the ovary.1 Malignant transformation of the various mature tissue components of a dermoid cyst is rare and the reported incidence is 0.17–1.4 %.2,3 Squamous cell carcinoma (SCC), arising from the ectodermal component, is the commonest form of malignant transformation accounting for >80 % of cases, followed by adenocarcinomas and carcinoid tumours.4
SCC in mature cystic teratomas is most commonly seen in postmenopausal women.4 There are no distinctive clinical features, tumour markers are often normal and pre-operative radiological diagnosis is difficult. Malignant transformation of an ovarian cyst is therefore difficult to predict, hence most cases are diagnosed postoperatively. 5 Tumours confined to the ovary usually have a better prognosis and fertility-sparing surgery is an option where appropriate.6 Conversely, patients with stage III or IV disease rarely survive five years.7
Our knowledge about these rare tumours is limited and based mainly on small case series and case reports and the optimal management of this condition is unclear. Whilst surgery is often performed initially, the appropriate adjuvant treatment following surgery is also unclear. The aim of this study is to present the experience of our centre in managing this rare tumour and review the key literature in this area. We hope this contributes to the evidence base and helps inform on the most appropriate management strategy for this rare type of tumour.
Patients and Methods
During a 24-year period (1986–2010) we retrospectively identified six patients treated for SCC arising in a mature cystic teratoma of the ovary at Velindre Cancer Centre, UK. We collected demographic and clinical outcome data from patient records and overall survival was defined from the date of diagnosis to the date of death. For each case we recorded patient characteristics, presenting symptoms, pre-operative tumour markers and imaging, tumour stage, treatment and outcome.
Results
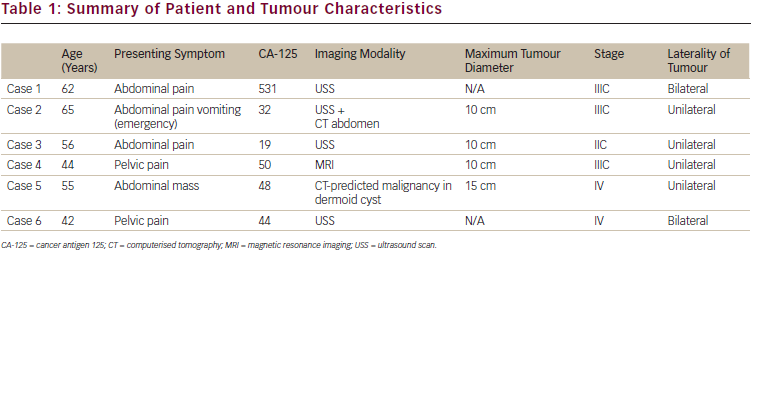
The age range of patients at diagnosis was 42–65 years with a median age of 56 years (see Table 1). Five women presented with chronic abdominal/ pelvic pain whilst one presented with acute abdominal pain and vomiting. Four of the patients had ultrasound scans, two had CT scans and one had a MRI scan as their pre-operative imaging study. Only one patient in our series, upon CT scan, was suspected pre-operatively to have malignant transformation in a dermoid cyst. The other patients had a diagnosis of a complex ovarian cyst. Pre-operative CA-125 was measured in all patients and was elevated in four patients and normal in two. Four patients had imaging studies available for review and the maximum tumour dimension range was 10–15 cm with a median of 10 cm.
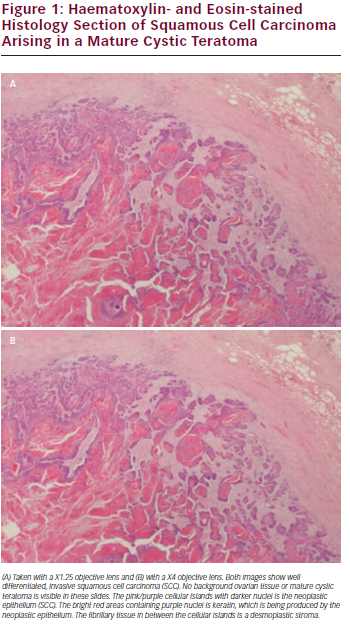
All six women underwent staging laparotomy and optimal cyto-reduction was achieved in only one patient (see Table 2). Two women had sub-total hysterectomies, one had a small bowel resection whilst another woman required a colostomy. None had lymphadenectomy as part of their surgical staging. One woman had stage IIC disease and was optimally debulked whilst the remaining patients all underwent surgical debulking but none achieved optimal cytoreduction. Of the patients who didn’t achieve optimal cytoreduction three had stage IIIc disease while the other two had stage IV disease. All patients had adjuvant carboplatin based chemotherapy and three women had paclitaxel and one had docetaxel additionally, as part of first-line chemotherapy. An example of a haematoxylin and eosin microscopic histology section from case 6 of an area of well-differentiated SCC arising in a dermoid cyst is shown in Figure 1.
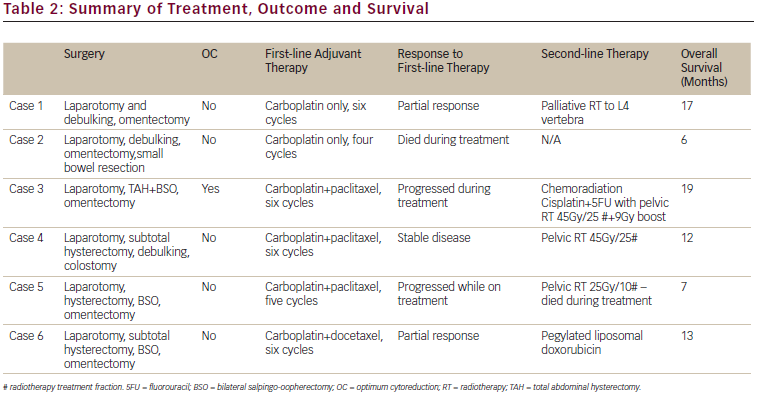
Two women achieved partial response following six cycles of chemotherapy. One had second line chemotherapy with pegylated liposomal doxorubicin for disease progression three months after completing first-line chemotherapy and died from disease progression 13 months from initial diagnosis. The second patient who responded had palliative radiotherapy to the lumbar spine for symptomatic management of progressive skeletal metastasis and survived 17 months from diagnosis. Of the other patients, one woman died after the fourth cycle of carboplatin chemotherapy from progressive disease, six months from initial diagnosis. One woman had stable pelvic disease after six cycles of combination chemotherapy and had a course of pelvic radiotherapy (45 Gy/25#). She had disease progression while on radiotherapy and survived a total of 12 months from diagnosis. The other two women had progressive disease on combination chemotherapy. One was switched to palliative pelvic radiation (25 Gy/10#) after five cycles of chemotherapy and died while on treatment, seven months from diagnosis. The other woman had chemo-radiation (cisplatin + 5-flurouracil with pelvic radiation 45 Gy/25#) for localised pelvic disease following six cycles of combination chemotherapy and had a partial response. She survived for 19 months, the longest in our series, from her initial diagnosis (see Table 2).
Discussion
Mature cystic teratoma of the ovary, often referred to as dermoid cysts, are benign and are one of the commonest ovarian tumours. They occur principally in premenopausal women with a mean age at diagnosis of 35 years.3 Malignant transformation of these tumours is rare and occurs in 0.17–1.4 % of cases.2,3 This usually occurs in postmenopausal women with studies showing a mean age of onset of 55 years,4 which compares well with the mean age of diagnosis in our patients. The average diameter of benign dermoid cysts is 6–7 cm2,3 while that of the malignant counterpart is 14 cm.4 The mean tumour diameter in our series is around 10 cm.
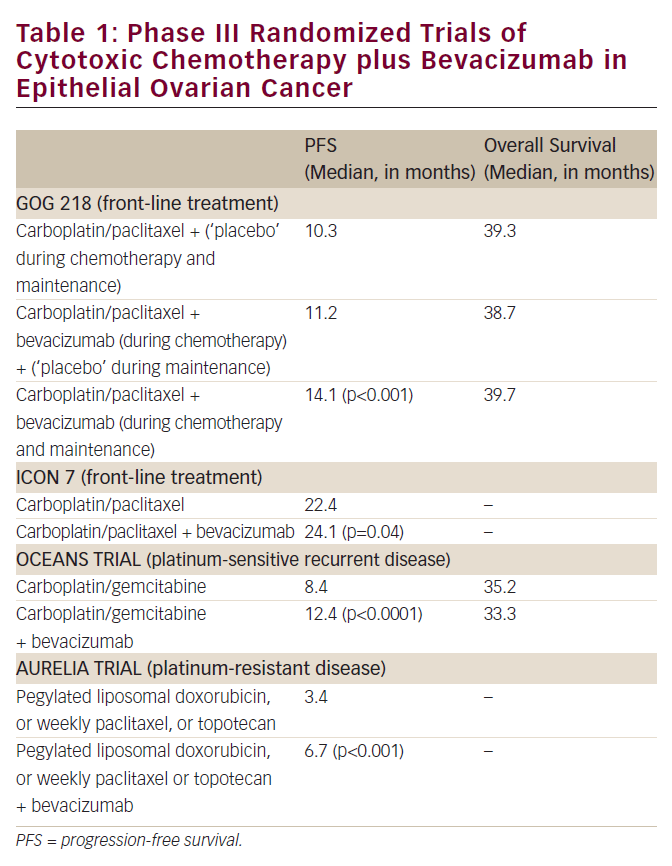
Measurement of serum tumour markers and imaging are two important elements in differentiating malignant from benign ovarian tumours. Our patients all had CA-125 levels checked pre-operatively and these were elevated in four out of six (67 %) patients in our series, which correlates well with a recent meta-analysis4 where 71 % of patients had elevated CA-125 levels. Higher CA-125 levels have been shown to be associated with adverse outcome;4 however, in our series the median survival was 12.5 months both for women with normal and elevated CA-125 levels. Other tumour markers have been reported to be elevated in the diagnosis of malignant transformation of mature cystic teratoma. Raised carcinoembryonic antigen (CEA) has recently been suggested as an important investigation when evaluating mature cystic teratoma. Raised CEA level has been demonstrated in a patient with adenocarcinoma in a mature cystic teratoma,8 but this was not checked in our patient series. Equally, an elevated CEA level has been reported as more useful than CA-125 and CA19-9 in the diagnosis of malignant transformation of mature cystic teratoma.7,8
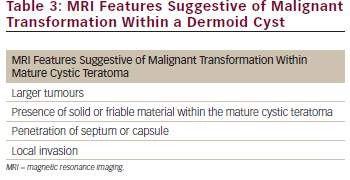
A diagnosis of malignant transformation of dermoid cyst was predicted in only one (17 %) patient in our series from preoperative imaging studies; which is a similar percentage to that reported in a recent case series.5 Mature cystic teratomas are relatively easily diagnosed using imaging modalities such as CT and MRI. Fat is usually present within mature cystic teratomas which gives appearance of low density on CT scan and high signal intensity on T1-weighted and T2-weighted MRI images.9,10 Fewer reports exist however regarding the radiological diagnosis of malignant transformation within a mature cystic teratoma. In one series of six cases examining the role of MRI in diagnosing these tumours, it was reported that the size of the tumour, penetration of the septum or capsule, invasion of adjacent organs and abundance of solid areas within cysts were features suggestive of malignant transformation within cystic teratomas (see Table 3).11 A recent case report has also demonstrated the value of MRI with fat suppression techniques to detect malignant transformation developing within a mature cystic teratoma.8
Though secondary debulking surgery has shown sustained remission in some reports none of our patients were deemed suitable for this intervention.5 In contrast to a meta-analysis,4 which showed 42 % of patients having tumour confined to one or both ovaries, all the patients in our study presented with extra-ovarian spread. Five out of six (83 %) of our patients had stage III or IV disease at surgical staging and though all patients underwent primary surgery only one (17 %) achieved optimal cytoreduction. None of our patients vhad lymphadenectomy whilst omentectomy was done routinely. A recent study suggested that lymphadenectomy might confer a survival advantage for patients with advanced disease whilst omentectomy did not affect overall survival.4
All our patients had platinum-based chemotherapy, which was based on our institutional protocol for advanced epithelial ovarian cancers (see Table 2). Two (33 %) patients demonstrated partial response to chemotherapy but had progressive disease at three and seven months, one patient (17 %) had stable disease while the other two (33 %) had progressive disease. Pegylated liposomal doxorubicin was tried as second line chemotherapy in one patient with progressive disease but no response was demonstrated. Meta-analysis of published data4 suggests that chemotherapy regimes containing alkylating agents might improve overall survival.
Two women had pelvic radiotherapy after combination chemotherapy for disease confined to the pelvis without any apparent benefit. One died midway through her treatment while the other had disease progression while on treatment. One patient demonstrated partial response to chemo-radiation following progression during first-line combination chemotherapy and she survived for longest in our series. Though results of a meta-analysis suggest that radiotherapy might affect survival adversely and increase morbidity4 chemo-radiation has not been evaluated in this setting and might be an option. None of our patients were alive at two years from initial diagnosis, the median survival being only 12.5 months, which reflects the poor prognosis associated with extra-ovarian spread of these tumours.
SCC arising from a mature cystic teratoma of the ovary is a rare malignancy and carries a very poor prognosis once the disease spreads beyond the ovary. Pre-operative diagnosis is difficult but MRI may be helpful in the diagnosis of malignant transformation. Tumour markers might be normal or only marginally elevated even in the presence of advanced cancer,
but some recent evidence suggests CEA level may be helpful when evaluating mature cystic teratomas. Certainly the possibility of malignant transformation should be considered in postmenopausal women with large tumours with solid components on magnetic resonance imaging. Optimal cytoreduction is difficult to achieve in advanced stage disease. Effective treatment and durable responses are, unfortunately, difficult to achieve in this condition but chemo-radiation may be of benefit in patients with localised pelvic disease, particularly as consolidation treatment after a response to first-line chemotherapy.