In vulvar neoplasia, substantial improvements in therapy and care have been achieved during the last decade.1 Nevertheless, even minor surgical interventions cause multiple symptoms and side effects that affect a woman’s quality of life.2 The term ‘vulvar neoplasia’ includes vulvar intraepithelial neoplasia (VIN) and vulvar cancer.3,4 Vulvar neoplasia is a rare condition, but with an increasing incidence in the last three decades in Europe and the US.5,6 The incidence of vulvar neoplasia in Germany and Switzerland is about two to seven per 100,000 women per year.7,8 Surgical treatment is the standard therapy.9,10 The prevalence of surgery-related complications, such as wound infections, is estimated to be between 5 % and 45 %.2,11
Complications are considered an adverse event of which a substantial number are preventable.12,13 Surgery-related complications in women with vulvar neoplasia result in a variety of physical and psychosocial problems, and contribute to high healthcare costs.2,14–16 Modified surgical procedures, such as the replacement of radical vulvectomy by less wide local excision, often means shorter hospital stays.17 Furthermore, oncology care in general is shifting to the outpatient setting.17 Thus, after discharge, women with vulvar neoplasia who have undergone surgical treatment are confronted with the need to assess, evaluate and manage surgery-related symptoms without the personal support of the in-patient care team.
Symptoms are defined as a patient’s perceived changes in biopsychosocial functioning, sensation or cognition,18,19 such as bleeding, shame and fear. A patient’s symptom experience includes two common dimensions: the cognitive dimension, which comprises symptom occurrence, frequency and severity; and the emotional dimension, which includes symptom distress.20
Humphreys et al.19 developed a theory of symptom management to try to understand how patients experience and manage symptoms and to provide healthcare professionals with guidance for selecting and evaluating clinical interventions, as well as to inform research. The model provides a framework for understanding the relationship between aspects of symptom experience, symptom-management strategies and patient outcomes. In a patient-centred healthcare approach, the theory provides appropriate guidance for treatment and care of patients with vulvar neoplasia. It is crucial to understand symptom experience in order to specify symptom assessment strategies and identify the focus for symptom management and attainable outcomes.18
Symptom assessment should be part of the general screening for adverse symptom events. This can be undertaken by collecting patientreported outcome (PRO) data.21 A PRO is any report of the patient’s health status that comes directly from the patient, such as his/her symptom experience.22 Systematic under-reporting and bias resulting from the use of standard summary methods have been described, potentially leading to treatment regimens appearing less toxic than they actually are. Patient reporting is valuable to detect the severity of adverse symptoms and permit timely treatment. There are limitations to both clinical reports and PROs. Therefore, assessment of both is the most accurate way to describe a patient’s status, rather than using traditional objective measurements alone. A combination of assessments allows for better supportive care.23 Basch21 emphasised the necessity of regularly including the patient’s voice by collecting PRO data as part of general screening for adverse symptom events. As clinicians often underestimate the severity of a patient’s symptoms, patient self-report can help to detect and prevent adverse events. When patient self-report of symptoms is standard practice, symptoms tend to be reported earlier in relation to their occurrence.21 Thus, a PRO instrument can add important information about the patient’s health status, the consequences of treatment and the effectiveness of interventions.22
In clinical practice, complications and symptoms after vulvar surgical treatment are an immense challenge for affected women because they are often discharged before their surgical wound has healed. Despite this, there is limited research about prevalence and risk factors for different types of short-term wound complications for this patient population and about women’s experiences during the first six months following surgical treatment. Furthermore, when our project was initiated, no structured symptom assessment instrument was available and there was little guidance for the assessment of symptom experience in women with vulvar neoplasia and surgical treatment. Nursing care and research have been hampered by a lack of effective symptom assessment measures following vulvar surgical treatment. An effective assessment should allow nurses to better assign management strategies and to be able to evaluate improvements in assessed symptoms. With the goal of improving care of women with vulvar neoplasia and surgical treatment, this international, multicentre research project aimed to develop a symptom assessment instrument for patients with vulvar neoplasia who have undergone surgical treatment.
Methods
We used a mixed-methods research approach.24 The project consisted of a series of quantitative and qualitative studies, with equal weighting of both methods, and used data of more than 180 women recruited from four Swiss (Zurich, Basel, Berne, St Gallen) and four German (Berlin, Dusseldorf, Freiburg, Munich) University Hospitals from 1997 to 2011. The first study of the research project was a cross-sectional investigation focusing on the clinical perspective. In studies 2 to 5, with a sequential exploratory two-phase strategy, our goal was to give voice to patients’ experiences by means of a qualitative approach. Their experiences were then systematically analysed in the quantitative component of the project.
Results
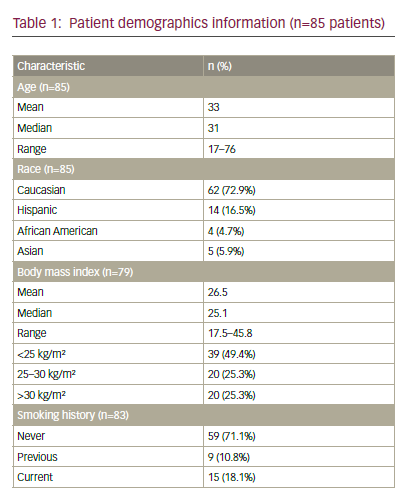
Our project started with a cross-sectional study on the period prevalence and risk factors for postoperative short-term wound complications in vulvar cancer patients in Switzerland. Data were collected retrospectively from medical records from 2007 to 1997 using an investigator-developed data collection instrument to assess risk factors preoperatively and perioperatively, and wound complications that occurred within the first 30 postoperative hospital days. The period prevalence of wound complications was 45.5 % (49/108 women), these women had at least one of eight assessed wound indications within the mean hospital stay of 11 days. The period prevalences for wound complications were: 31.5 % for dehiscence, 12 % for haematoma, 6.5 % for necrosis, 5.6 % for infection, 4.6 % for seroma, 1.9 % for lymph cysts, 1.9 % for malignant wounds; no patients experienced disturbed tissue formation. Two of the 14 risk factors examined, the type surgical therapy (odds ratio [OR] 2.6; 95 % confidence interval [CI] 1.34–5.14) and inguinofemoral lymphadenectomy (OR 3.0; 95 % CI 1.03–8.76), were significantly associated with short-term wound complications. The results of this retrospective study showed a high prevalence of shortterm wound complications and supported the need for systematic wound assessment and early risk management, especially after haemivulvectomy, radical vulvectomy and inguinal lymphadenectomy.25
In order to understand the women’s perspective, we then conducted a qualitative study exploring the symptom experience in 20 women with vulvar precancer or cancer and surgical treatment in one Swiss and two German University Hospitals. Narratives showed eight key themes composing the essence of women’s symptom experience: delayed diagnosis, disclosed disease, disturbed woman’s self-image, changed vulva care, experienced wound-related symptoms, evoked emotions, affected interpersonal interactions and feared illness progression. The identified themes were consistent across different surgical procedures. The pattern present in all narratives was that women experienced a lack of information in terms of the above interrelated themes and that all of them used strategies to handle their situation, which affected their distress level. The communication, assessment and treatment of symptoms were hampered by the tendency of society and the health system to overlook these symptoms and leave them in the realm of the unspeakable.26
In parallel, we focused on the perspective of women with VIN during their illness trajectory by conducting a secondary data analysis of data collected during the qualitative study. Eight narratives showed women’s experiences during their course of illness occurred in five phases: There is something unknown; One knows what it is; It is treated and should heal; It has effects on daily life; and Meanwhile it works. These phases showed that many women: had a late or incorrect diagnosis; did not know what they suffered from despite having a diagnosis; experienced high uncertainty in decision-making during treatment, experienced a physical and psychosocial functioning effect after VIN; learned over time to deal with their illness. Central for these women during their course of illness was a sense of ‘Hope and Fear’. It reflected the fear of recurrence but also the trust in healing. Women’s experiences were particularly influenced by feelings of ‘embarrassment’ and by ‘dealing with professionals’. Current care seems to lack adequate support for women with VIN to manage these phases.27
The next step was the development of a PRO instrument for WOMen with vulvAr Neoplasia (WOMAN-PRO) according to the PRO guidelines,22 and based on literature searches, expert feedback (n=9) and patient interviews (n=20). Thirty-seven items were pilot-tested with patients (n=6) and experts (n=6). The revised 36 items were trialled with patients (n=4). Participants were recruited from one Swiss and two German University Hospitals. To our knowledge, this is the first PRO measure designed specifically to assess postvulvar surgery symptoms, informational needs and related distress after hospital discharge. The revised WOMAN-PRO showed an excellent item and scale Content Validity Index (CVI=1.0) and has the potential to support women in recognising, assessing and evaluating the seriousness of postsurgical symptoms and in communicating their symptom experience to healthcare providers. This can reduce women’s uncertainty about symptoms and reassure their decision-making about when to seek healthcare provider evaluation. It can provide clinical experts with systematic information about key symptoms from a patient perspective, and women’s unmet informational needs.
Finally, we identified the occurrence and distress associated with postsurgery symptoms of women with vulvar neoplasia measured with the WOMAN-PRO. The study was a prospective cross-sectional survey conducted in eight hospitals in Switzerland and Germany. We conducted an interim analysis with 54 outpatients. They rated the occurrence of each of 31 symptoms, and the degree to which the symptoms distressed them. The average number of symptoms reported per patient was 20 (standard deviation [SD] 5.02) with a range of 10 to 31 symptoms. The three most prevalent wound-related symptoms were ‘swelling’ (n=46), ‘drainage’ (n=46) and ‘pain’ (n=43). The three most prevalent difficulties in daily life were ‘sitting’ (n=52), ‘wearing clothes’ (n=48) and ‘carrying out my daily activities’ (n=43). ‘Tiredness’ (n=51), ‘insecurity’ (n=44) and ‘feeling that my body has changed’ (n=42) were the three most prevalent psychosocial symptoms. Symptom-related distress was rated on a scale from 0 to 3 from ‘not at all’ = 0, a little bit = 1, quite a bit = 2 and very much distressing = 3. The most distressing symptoms were ‘sitting’ (mean [M]=1.98; SD 0.90), ‘carrying out my daily activities’ (M=1.79; SD 0.89),and ‘open spot’ (e.g. opening of skin or suture) (M=1.79; SD 0.92), which were on average reported to be ‘quite a bit’ distressing. Based on the interim analysis, we also examined the reliability of the instrument using a Cronbach’s alpha coefficient. For the items representing woundrelated symptoms and difficulties in daily life alpha was 0.70, and it was 0.87 for items representing psychosocial symptoms. An alpha of 0.70 or above reflects adequate reliability.28 The WOMAN-PRO data collected in this study show high symptom prevalence and distress, call for a comprehensive symptom assessment and may allow identification of areas for symptom management. If the results of further psychometric testing are promising, the WOMAN-PRO will provide a useful outcome measure for clinical trials.
Discussion
The results of the research project contributed to the evidence on women with vulvar neoplasia and surgical treatment. First, it added knowledge to the ability of support nurses and physicians to identify patients at risk of postsurgical wound complications. Second, it provided a conceptual model of symptom experience in affected women. Third, it established evidence to help understand the VIN patient’s experiences during her illness trajectory. Fourth, the development of the WOMAN-PRO instrument with a good content validity and reliability has the potential to contribute to a valid assessment of symptoms, informational needs and related distress in women diagnosed with vulvar neoplasia and treated surgically. Fifth, WOMAN-PRO data showed a high symptom prevalence and distress, and provided preliminary evidence that the WOMAN-PRO instrument offers a feasible, targeted screening instrument to support systematic symptom assessment.
Future Research Implications/Perspectives
Based on our findings, further studies need to include a larger sample so that additional psychometric properties of the WOMAN-PRO can be examined. We only examined women’s symptom experiences and distress during the first seven days after discharge following vulvar surgery. Future studies should examine the ability of the WOMAN-PRO to detect changes in women’s symptom experience over time following surgery. In general, nursing research needs to focus on intervention studies to be more applicable for clinicians and patients.29–31 It is important for intervention studies to include when possible PRO self-reporting measures to monitor subjective adverse events and assess specific treatment benefits from the patient perspective.32,33 This may improve the quality and completeness of disease specific intervention outcome data, provide a more comprehensive picture of the patient experience and improve the efficiency of clinical operations.34 Based on the evidence discussed above, we plan to develop and test a patient self-management support intervention for women with vulvar neoplasia and continue to test the psychometric properties of the WOMAN-PRO instrument in future studies. Guided by the UK Medical Research Council’s Complex Intervention Framework,35 development and testing a patient self-management support intervention requires the following three steps:
- A meta-synthesis of qualitative studies describing aspects of self-management in women with vulvar neoplasia needs to be conducted.36 This should result in a comprehensive understanding of informational and educational needs, self-management tasks and skills needed by affected women and their families. Then it is necessary to decide which needs can be translated into a self-management support intervention for women with different disease stages, therapies and co-morbidities.
- A systematic literature review is required to elaborate the most effective self-management support intervention in terms of improving patient outcomes in the field of gynecologic oncology since, based on our knowledge, no such interventions exist in the vulvar neoplasia literature. In addition, current evidence about the effective implementation of research interventions in clinical practice should be considered.37,38
- The newly developed self-management support intervention with a specified dose and duration should be tested in a pilot study to assess its effectiveness and feasibility as well as to understand the change process.35
To allow the simultaneous examination of the newly developed selfmanagement support intervention and the psychometric testing of the WOMAN-PRO instrument, validated tools should be included in the planned pilot study to measure symptom occurrence and distress. In addition, in order to recruit a large enough sample size to ensure acceptable statistical power to perform analyses on subgroups, e.g. women with different disease stages, therapies and co-morbidities, a multicentre study will be required.
Implications and Perspectives for Practice
In addition to the implications for future research, we believe that this study also has implications for clinical practice. The high prevalence of symptoms and the finding that symptoms that are less common may, nonetheless, be distressing for the women who experience them, supports the need for the systematic assessment of symptoms and their related distress in patients follow surgical treatment of vulvar cancer. Given this, we recommend that clinicians use and evaluate the new PRO self-report instrument as a major element of follow-up care in women with VIN and vulvar cancer.
The shift of care to outpatient settings, short hospital stays after surgical treatment and treatment by different healthcare providers present challenges for healthcare.39 Outpatient care and shorter hospital stays may reduce time for counselling and increase risk of unsystematic assessment and intervention. Inconsistencies in the care provided among different healthcare providers call for a systematic assessment of symptoms and their related distress.39,40 While research supports the importance of including patient reports when assessing symptoms of adverse events,41–43 Cirillo et al. observed better agreement between nurse and patient-reported adverse event symptoms than patient and physician reports.44 Therefore, nurses can play an important role in collecting symptom data related to adverse treatment events and prevent complications or report early signs of recurrence.44
In the German-speaking context, interdisciplinary follow-up visits may improve recognition of early signs of recurrence and adverse events. Nurses and physicians play a major role in symptom assessment and management in women with vulvar neoplasia and their families. Based on our research and that of others,41,43,45 we recommend the incorporation of PRO self-reports into the follow-up care in this patient population to facilitate communication, decision-making, symptom assessment and management. Clinicians should be educated in the delivery of individualised evidence-based care based on PRO data. The synthesis of PRO information with clinician’s objective observations will improve care for women who have vulvar neoplasia and surgical treatment. We suggest nurse-led follow-up consultations complementary to physician appointments to allow adverse symptom event monitoring. Whether vulvar-specific instruments will improve the completeness or interpretability of adverse symptom event data will be evaluated and reported in the future.32
Conclusion
This research project added knowledge on complications, symptoms and associated distress of women with vulvar neoplasia and surgical treatment. It confirmed the need for further research on implementation strategies for comprehensive symptom assessment and the identification of areas in symptom management to support early recognition of symptoms and decrease symptom related distress in women with vulvar neoplasia and surgical treatment.46
Presentation
Research results were presented in part at the European College for the Study of Vulval Disease (ECSVD) 8th Congress and Post-Graduate Course in Munich, 2010 (16–18 September 2010), and in part at the European Multidisciplinary Cancer Congress in Stockholm, Sweden, (23–27 September 2011). The preliminary report of this article with a sample size of 54 women was part of a dissertation thesis published by the University of Basel (reference 39: 10.5451/unibas-005976880). According to their declaration, the copyright is held by the first author.