Endometrial carcinoma is the most common malignancy of the female genital tract, accounting for more than 42,000 cases annually in the US (6% of new cancer cases).1 Worldwide, it is the seventh most common cancer in women, with over 200,000 cases annually.2 Fortunately, most women present with dysfunctional vaginal bleeding or spotting—an early sign of endometrial pathology—and ultimately will be cured with surgical therapy. Unfortunately, more than 7,500 die of advanced or recurrent endometrial carcinoma every year.1 Endometrial carcinomas are classified as type I or type II.3,4 Type I tumors evolve in an estrogenrelated manner, are associated with endometrial hyperplasia, and express steroid hormone receptors. The usual pathologic phenotype is an endometrioid adenocarcinoma, which tends to be well differentiated and of low grade. By contrast, type II tumors—classically characterized as serous or clear-cell carcinomas—arise in atrophic endometrium in a manner unrelated to estrogen exposure. These tumors are usually negative or weakly positive for steroid hormone receptors, and are typically poorly differentiated and of high grade. In general, type II tumors are characterized by a more aggressive clinical course than type I tumors.3
The current treatment of endometrial carcinomas requires multimodal therapy and depends on the stage at presentation, histologic grade, and type. Surgery alone or with radiation therapy, chemotherapy, or endocrine therapy are all utilized in the front-line management of this disease. For women presenting with high-risk features (serous or clear cell, grade 3, deeply invasive to the myometrium, and nodal involvement), a combination of surgical staging followed by chemotherapy with or without radiation therapy is often employed. Chemotherapy as a First-line Treatment
The use of chemotherapy has evolved over the past decade. Once used for women who progressed following surgery and radiation, several randomized trials have supported its role following surgical treatment. The Gynecologic Oncology Group (GOG) 122 study randomized 396 patients with stage III/IV endometrial carcinoma who had undergone total abdominal hysterectomy to whole-abdominal radiotherapy (WAR) or chemotherapy with doxorubicin and cisplatin (AP) every three weeks for eight cycles.5 With a median follow-up of 74 months, AP significantly improved progression-free survival (PFS: hazard ratio [HR] 0.71, 95% confidence interval [CI] 0.55–0.91) and the stage-adjusted death hazard ratio (HR 0.68, 95% CI 0.52–0.89) compared with radiation therapy. Estimated five-year PFS was 38% for WAR compared with 42% with AP, with five-year overall survival (OS) estimated at 42 and 53%, respectively. The Japanese GOG 2033 trial randomized women with stage IC–III tumors to whole pelvic radiation or a combination of cisplatin, doxorubicin, and paclitaxel (CAP) every four weeks for at least three cycles.6 There was no difference between radiation and chemotherapy in five-year PFS (84 versus 82%; p=0.726) and OS (85 versus 87%; p=0.462).
In the subgroup analysis, women at high to intermediate risk were defined as having stage IC with age >70 years or grade 3 tumors, or by stage II or IIIA (positive cytology) with >50% myometrial invasion. These women had significantly better PFS (84 versus 66%; p=0.024) and OS (90 versus 74%, p=0.006) with chemotherapy than radiation.6
GOG 184 randomized 552 women with advanced disease who underwent surgical debulking and adjuvant radiation therapy (with dosing based on the extent of disease) to AP or CAP every three weeks for six cycles.7 At three years, recurrence-free survival did not differ between the CAP and AP arms (64 versus 62%); the overall hazard with CAP compared with AP stratified by stage was 0.90 (95% CI 0.69–1.17). In a subgroup analysis, CAP was associated with a 50% reduction in the risk for relapse or death compared with AP in women with gross residual disease at enrollment (HR 0.50, 95% CI 0.26–0.92).7 Of note, the CAP regimen was associated with more frequent and severe hematologic toxicity, sensory neuropathy, and myalgia.
GOG 209 has been designed to build on this experience by evaluating whether a less toxic regimen (paclitaxel/carboplatin) is equivalent or superior to CAP. In this trial, women with stage III or IV endometrial cancer are randomized to paclitaxel/carboplatin or CAP every three weeks for seven cycles. Radiation therapy is allowed, provided it is completed at least four weeks prior to the start of chemotherapy. The results are eagerly anticipated.8
Chemotherapy for Relapsed Disease
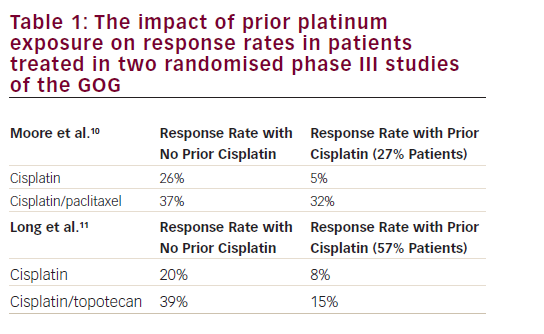
There are currently no US Food and Drug Administration (FDA)-approved agents in this setting. With more frequent use of cisplatin, carboplatin, doxorubicin, and paclitaxel in the adjuvant setting, women who have disease recurrence or develop metastastic disease face limited choices for additional treatment, with few active agents identified following formal phase II evaluation (see Table 1). Often, the emphasis of treatment is on palliation of symptoms and disease control.Pegylated Liposomal Doxorubicin
PLD encapsulates doxorubicin in a liposome coated with polyethylene glycol for improved stability. This newer formulation results in prolonged circulation of doxorubicin in the bloodstream, a longer half-life, and a smaller volume of distribution. Delivery of the active drug to the tumor bed is enhanced through leaky tumor neo-vasculature, with reduced exposure of healthy tissues with normal blood vessels.11,12
This formulation alters the toxicity profile of PLD compared with doxorubicin; it is associated with a diminished risk of cardiac toxicity, bone marrow suppression, nausea, vomiting, and alopecia. The toxicity of PLD is predominantly dermatologic: stomatitis and palmar-plantar erythrodysesthesia tend to be dose- and schedule-dependent and can occur in up to 48% of patients.12
There have been a limited number of clinical trials that have looked at the efficacy of PLD in advanced or recurrent endometrial cancer as a single agent or in combination. The largest study was reported by Muggia et al. for the GOG. That study enrolled 45 women with previously treated endometrial cancer, 32 of whom had received prior doxorubicin. PLD was dosed at 50mg/m2 every four weeks. The overall response rate (ORR) was 9.5% and only four partial responses were seen. The median survival was 8.2 months.13 A smaller prospective study treated 19 women with PLD at 40mg/m2. The response rate was 21%, but no survival data were reported.14 The GOG conducted another phase II trial of PLD in the first-line setting in advanced or recurrent endometrial carcinoma. Fifty-six women were treated with PLD 40mg/m2 every four weeks. There were two complete responses and four partial responses for an ORR of 11.5%. An additional 31 patients had stable disease.15
More recently, a phase II trial from Italy treated 19 women with chemonaïve disease using PLD 40mg/m2 every four weeks with an ORR of 36% and a 46% stable disease rate.16 The adverse effects observed in these studies were moderate and in keeping with the known toxicity of PLD. Grade 3–4 anemia occurred in 11–26% of patients, neutropenia in 5–16%, and palmar-plantar erythrodysesthesia in 0–9%. Cardiotoxicity was observed in women with and without prior anthracycline exposure. PLD was evaluated in combination with carboplatin in three phase II trials. In the largest trial, 42 women received carboplatin (area under the curve [AUC] 5) and PLD 40mg/m2 every four weeks. The ORR was 59%, with a PFS of 52 weeks.17 The two other phase II trials using PLD and carboplatin as first-line therapy showed response rates of 44 and 32%.18,19 In all three of the above studies, treatment was well tolerated and no treatment-related cardiac toxicity was observed. In another report, 17 chemonaïve patients were prospectively treated with PLD 30mg/m2 and weekly paclitaxel 75mg/m2. A response rate of 47% was observed.20
Chemotherapy for Uterine Papillary Serous Carcinoma
Uterine papillary serous carcinoma (UPSC) is a more aggressive type of endometrial carcinoma, accounting for 5–10% of all cases of endometrial carcinoma but contributing up to 39% of cancer deaths.21,22 UPSCs are high-grade tumors that are often associated with lympho-vascular invasion and a high rate of nodal metastases and intraperitoneal dissemination that is not predicted by the degree of myometrial invasion.21,23 Retrospective studies have shown an inverse relationship between survival and the volume of residual disease after cytoreductive surgery, even in women with metastatic disease.24,25 There is evidence that adjuvant platinum- and taxane-based therapy reduces recurrences and improves survival, even in early-stage disease.26,27 Although previously considered relatively resistant to chemotherapy, response rates of advanced or recurrent UPSC to doxorubicin and cisplatin have been shown to be similar to the response rate of the endometrioid variant in compiled data from four phase III GOG trials.28 The ORR of firstline doxorubicin, as seen in the single agent arm of the phase III trial GOG 107, was 25%.29 Given the similarities between recurrent serous ovarian and uterine carcinomas, recurrent UPSC is commonly treated with drugs that have shown activity in recurrent ovarian carcinoma. PLD in recurrent ovarian cancer is moderately active, with response rates around 20%, and is generally well tolerated.30Data on the efficacy of PLD in advanced or recurrent UPSC are almost non-existent and generally consist of single-institution case reports. In one such report, three of nine patients had a response after treatment with first-line PLD and weekly paclitaxel.20 In another report, 23 patients with recurrent UPSC who were treated with PLD were retrospectively compared with 24 patients with recurrent endometrioid uterine carcinoma treated with PLD. Response rates were not reported, but PFS and OS were similar at 4.6 and 9.8 months and 4.7 and 9.6 months for serous and endometrioid uterine carcinoma, respectively.31
Conclusion
The available, though limited, data on PLD in advanced or recurrent endometrial cancer are not encouraging, suggesting inferior activity as a single agent in the first-line setting and limited effectiveness in patients who have received prior chemotherapy. On the other hand, the rate of tumor response achieved with the combination of PLD and carboplatin in previously untreated patients compares favorably with paclitaxel-containing regimens commonly used in first-line therapy, and the regimen was well tolerated. More studies will be necessary to better define the place of this regimen in the treatment of advanced or recurrent endometrial cancer.
As for the use of PLD in the treatment of advanced or recurrent UPSC, in the few studies available efficacy data were not broken down by histologic type. The rate of response to chemotherapy for UPSC has been shown to be equivalent to that of endometrioid carcinoma. Before more efficacy data specific for UPSC become available, it is therefore reasonable to consider that the activity of PLD in UPSC is no better than what the reports described above have shown for all endometrial tumor types combined. ■