Cervical cancer (CC) remains one of the most frequent cancers in women, representing the fourth cause of neoplasia in women in terms of incidence, and has a high lethality rate.1 Indeed, worldwide 341,831 women die each year because of this disease, with a mortality rate that varies across different countries, with, for example, less than two per 100,000 in Western countries such as Australia and New Zealand to more than 22 per 100,000 in Africa.1
CC is mainly attributable to human papillomavirus (HPV) infection. Thanks to the introduction of specific vaccination and screening programmes for HPV, mortality has decreased by 75% in industrial countries over the past 50 years.2 Nevertheless, the worldwide incidence of CC is still significant, with approximately 604,000 new cases per year.1 Patients diagnosed in the metastatic stage have a poor prognosis, with a 5-year survival rate of only 17%.3 Early-stage disease, which consists of stages from I to IIA, can usually be managed by surgery alone whereas locally advanced stages, including stages from IIB to IVA, are usually treated by cisplatin-based chemoradiotherapy.4 However, the risk of recurrence is 11–22% and 28–64% for early and locally advanced stages, respectively.5 In cases of relapsed or de novo metastatic CC, the prognosis remains poor, with an overall survival (OS), at diagnosis, ranging from 5 to 16 months.6 Cisplatin-based chemotherapy, alone or in association with paclitaxel, represents the gold standard first-line treatment for persistent, recurrent or metastatic CC, with an overall response rate (ORR) of 13% in monotherapy and 36% in platinum-based doublet.7,8 When associated with cisplatin, paclitaxel demonstrated the same efficacy but less toxicity compared with topotecan, gemcitabine or vinorelbine.8 In the recurrent setting, carboplatin–paclitaxel demonstrated non-inferiority compared with cisplatin–paclitaxel doublet in patients pretreated with cisplatin.9 In 2014, the addition of bevacizumab to cisplatin–paclitaxel chemotherapy showed an improvement of OS, with 16.8 months in the bevacizumab chemotherapy arm compared with 13.3 months in the chemotherapy alone arm (hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.62–0.95; p=0.007), leading the bevacizumab regimen to become the new standard first-line therapy in the metastatic setting.10,11 At the time of writing, there is no evidence that further treatments after first line improve OS compared with best supportive care. However, women in this setting are young and symptomatic for their disease; treatment options that confer improvement in disease-related symptoms, quality of life and prolongation of progression-free survival (PFS) are worthwhile. Several phase II studies evaluating cytotoxic or targeted agents as second-line treatment have shown response rates lower than 10%.12–21 The first reported retrospective series of patients treated at the Royal Marsden Hospital (London, UK), from 2004 to 2014, with second-line systemic treatment for recurrent or metastatic CC demonstrated an ORR to second-line therapy of 13.2%, a median PFS of 3.2 months (95% CI, 2.1–4.3) and median OS of 9.3 months (95% CI, 6.4–12.5).22
Based on the promising results of immune checkpoint inhibitors (ICIs) in other solid tumours, anti-programmed cell death-1 (anti-PD-1) and anti-programmed cell death-ligand 1 (anti-PD-L1) are under investigation in advanced CC. Indeed, the KEYNOTE-826 trial addresses the question of whether adding pembrolizumab to platinum-based chemotherapy would improve efficacy as first-line of treatment for advanced CC.23
The KEYNOTE-826 trial
Methods
The KEYNOTE-826 trial is an international, multicentre, double-blind phase III trial.23 Patients with persistent, recurrent or metastatic adenocarcinoma, adenosquamous or squamous cell carcinoma of the cervix who had not previously been treated with systemic chemotherapy and had no prospect of curative intent, were eligible for the trial. Patients were randomly assigned in a 1:1 ratio to receive pembrolizumab 200 mg or placebo every 3 weeks for up to 35 cycles plus the investigator’s choice of a platinum-based chemotherapy regimen (paclitaxel 175 mg/m2 plus either cisplatin 50 mg/m2 or carboplatin area under the concentration–time curve [AUC], 5 mg/mL/min) with or without bevacizumab 15 mg/kg, again at the investigator’s discretion. The dual primary endpoints were PFS and OS, tested sequentially in patients with a PD-L1 combined positive score (CPS) of ≥1 (PD-L1 immunohistochemistry [IHC] 22C3 pharmDx assay), in the intention-to-treat (ITT) population, and in patients with a PD-L1 CPS of ≥10.
Results
Patient characteristics
All results come from the first interim analysis. Between November 2018 and January 2020, 617 patients were randomly assigned to receive either pembrolizumab plus chemotherapy with or without bevacizumab (pembrolizumab group 308 patients) or placebo plus chemotherapy with or without bevacizumab (placebo group 309 patients).23 Bevacizumab was prescribed to 63.6% of patients in the pembrolizumab group and to 62.5% of patients in the control group. Overall, 56.4% had received previous chemoradiotherapy with or without surgery, and 19.8% had previously untreated metastatic disease at trial entry. The majority of the patients had squamous cell carcinoma (76.3% in the pembrolizumab group and 68.3% in the placebo group); 548 patients had a PD-L1 CPS ≥1 (273 in the pembrolizumab group and 275 in the placebo group) and 11% of patients in each arm had a PD-L1 CPS <1.
Efficacy
After a median follow-up of 22.0 months, for the 548 patients with a PD-L1 CPS ≥1, the median PFS was 10.4 months in the pembrolizumab group and 8.2 months in the placebo group (HR, 0.62; 95% CI, 0.50–0.77; p<0.001); in the ITT population, comprising 617 patients, PFS was 10.4 months and 8.2 months, respectively (HR, 0.65; 95% CI, 0.53–0.79; p<0.001). For the 317 patients with a PD-L1 CPS ≥10, PFS was 10.4 months and 8.1 months, respectively (HR, 0.58; 95% CI, 0.44–0.77; p<0.001). Interim analysis for OS showed a survival rate of 53.0% at 24 months in the pembrolizumab group and of 41.7% in the placebo group (HR, 0.64; 95% CI, 0.50–0.81; p<0.001). OS was 50.4% and 40.4%, respectively (HR, 0.67; 95% CI, 0.54–0.84; p<0.001) for patients with PD-L1 CPS ≥1, and 54.4% and 44.6%, respectively (HR, 0.61; 95% CI, 0.44–0.84; p=0.001) for patients with PD-L1 CPS ≥10.23
The ORR was improved in the pembrolizumab arm compared with the placebo arm, regardless of the CPS score: 69.6% versus 49.1% in the CPS ≥10 group; 68.1% versus 50.2% in the CPS ≥1 group; and 65.9% versus 50.8% in the ITT population. More complete responses (CRs) were observed in the pembrolizumab arm: 22.2% versus 11.3% in the CPS ≥10 group; 22.7% versus 13.1% in the CPS ≥1 group; and 21.4% versus 12.9% in the ITT population. The median duration of response was longer in the pembrolizumab group than in the placebo group: 21.1 versus 9.4 months in the CPS ≥10 group; 18 versus 10.4 months in the CPS ≥1 group; and 18 versus 10.4 months in the ITT population.23
Safety and quality of life
The median treatment duration was 10.0 months in the pembrolizumab group and 7.7 months in the placebo group.23
Grade 3–5 adverse events (AEs) occurred in 81.8% of the pembrolizumab group and 75.1% of the placebo group. The most common severe grade 3–5 AEs were anaemia (30.3% in the pembrolizumab group and 26.9% in the placebo group) and neutropenia (12.4% and 9.7%, respectively). Only hypothyroidism (incidence 18.2% versus 9.1%) and leukopenia (12.1% versus 7.1%) were noted as AEs with an incidence of 10% or greater risk in the pembrolizumab group. Importantly, no grade 3–5 AEs with an incidence higher than 5% were observed in the experimental arm.23
Potentially immune-mediated AEs were observed in 33.9% of the patients in the pembrolizumab group and in 15.2% of those in the placebo group, with 11.4% and 2.9% of grade 3–5 AEs, respectively. The most important of these was hypothyroidism, occurring in 18.2% of patients in the pembrolizumab group versus 9.1% in the placebo group. Of note, one patient in the pembrolizumab arm died because of an immune-mediated AE (encephalitis).23
Interestingly, discontinuation of any agent due to AEs occurred in 37.5% in the pembrolizumab group and 26.5% of the patients in the placebo group, but quality of life was improved in the experimental arm. Indeed, time to deterioration in the EuroQol health status measure, EQ-5D-5L, visual analogue scale score was higher with pembrolizumab than with placebo (12-month estimate of patients free from deterioration, 58.2% versus 44.8%, respectively; HR, 0.75; 95% CI, 0.58–0.97).23
Conclusion
This large, well-designed, randomized international phase III trial provides a high level of evidence of the efficacy of pembrolizumab associated with chemotherapy, in terms of OS in the first-line setting.23 These data will be practice changing and the benefits observed on survival are much needed in this vulnerable population. CC has been the ‘poor relation’ in gynae-oncology and in oncology in general, but this study provides further support for immunotherapy as the new cornerstone of its management. However, some questions persist and the utility of this regimen should be explored further to establish whether immunotherapy could be used even earlier in CC, in localized disease.
Why does anti-PD-(L)1 work in cervical cancer?
Preclinical rationale
As described above, anti-PD-(L)1 ICIs, such as pembrolizumab, have produced interesting results in clinical trials, confirming the preclinical rationale. In fact, 90% of CCs are induced by HPV infection.24 At the beginning of infection, acute inflammation and immune recognition are inhibited by HPV-positive cells, leading to an immune escape, which in turn results in viral persistence. This inflammation persists until it becomes chronic and allows the interaction between neoplastic cells and tumour microenvironment promoting carcinogenesis.25 In CC cells, HPV-16E7 viral protein is not only responsible for lymphocyte dysfunction, but also induces overexpression of PD-L1, which can thus be used as a biomarker of HPV infection of the cervix.26 PD-L1 is significantly upregulated in CC within CD8+ lymphocytes and is detectable by IHC in tumour cells,27 mainly in squamous cell carcinomas (54% versus 14% in adenocarcinomas).28 Researchers in the Cancer Genome Atlas project performed a comprehensive analysis of the invasive CC genome. They identified amplifications in multiple immune checkpoint targets in these tumours, as well as PD-L1, encoded by the CD274 gene.29,30 The pathogenic nature of the disease, the contribution of genes controlling the immune reaction and the high score of immune infiltration provide the rationale for evaluation of ICIs in CC.
Clinical trials
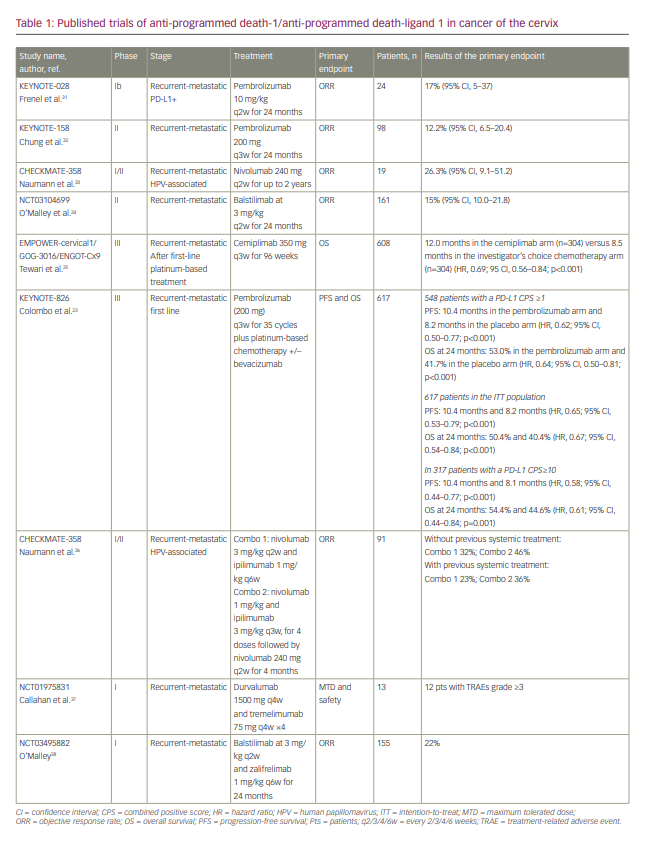
The anti-PD-(L)1 ICIs were first evaluated as monotherapy in pan tumours first-in-man trials (Table 1).23,31–38 The PD-1 inhibitor pembrolizumab was evaluated in two trials, KEYNOTE-028, a phase Ib basket trial,31 and KEYNOTE-158, a phase II trial.32 The KEYNOTE-028 trial involved recruitment of 24 patients with PD-L1-positive advanced CC, after progression under prior systemic therapy; ORR was the primary endpoint and was achieved in 17% of patients.31 The KEYNOTE-158 trial recruited 98 patients, 82 of whom had a positive PD-L1 CPS (≥1); the primary endpoint of ORR was achieved in 12.2% of patients, including three with a CR and nine with a partial response (PR).32 The CHECKMATE 358 trial, a phase I/II trial recruiting HPV-associated tumours, assessed the efficacy of nivolumab, another anti PD-1 inhibitor.33 The primary endpoint of ORR was reached in 26.3%, including three CRs and two PRs among the 19 patients enrolled with CC.33 Afterwards, on the wave of these promising response rates, anti-PD-(L1) blockade was compared with standard of care in the EMPOWER-cervical1/GOG-3016/ENGOT-Cx9 phase III trial.39 In this trial, patients with recurrent and metastatic CC resistant to platinum-based chemotherapy, were randomly assigned to receive cemiplimab, an anti-PD-1, or mono-chemotherapy according to the investigator’s choice. After a median follow-up of 18.2 months, the primary endpoint of OS was significantly improved in the cemiplimab arm: 12 months versus 8.5 months in the chemotherapy arm (HR, 0.69; 95% CI, 0.56–0.84; p=0.00011). No major AEs were noted, especially no new immune-related AEs. In light of these results, which are probably practice changing, one question remains: does anti-PD-(L)1 treatment in monotherapy provide benefit for all patients with CC relapsing after platinum-based chemotherapy?
Is PD-L1-negative status an inappropriate candidate?
Despite the interesting results of early-phase trials and more recently with the two randomized phase III trials EMPOWER-cervical 1/GOG-3016/ENGOT-Cx9 and KEYNOTE 826, no biomarkers have been identified to select patients most likely to benefit from ICI.
Thus, what is the predictive value of PD-L1 status in the response of pembrolizumab in CC? PD-L1 protein expression can be used to evaluate the efficacy of ICI as it reflects the activation of the immune cells induced by the interferon gamma pathway.40,41 Furthermore, the predicted effect of immunotherapy can be evaluated by using PD-L1 expression as a biomarker. PD-L1 expression is assessed via IHC on formalin-fixed paraffin-embedded tissues. Two major scores are used to evaluate PD-L1: the CPS and the tumour proportion score (TPS). The CPS is determined by IHC with the anti-PD-L1 mouse monoclonal antibody, 22C3 (pharmDx; Agilent Dako, Santa Clara, CA, USA), and is calculated by the number of PD-L1 staining cells (tumour cells, lymphocytes, macrophages), divided by the total number of viable tumour cells, multiplied by 100. In the same way, the TPS is established via IHC with the 22C3 antibody and is defined as the percentage of viable tumour cells with partial or complete PD-L1 membrane staining at any intensity.42 The 22C3 antibody presents robust immune cell staining, with staining similar to SP263 (Ventana Medical Systems, Inc., Tucson, AZ, USA) and 28-8 (pharmDx; Agilent Dako) antibodies, but higher staining than the SP142 (Ventana medical Systems Inc.) antibody.43,44
The CPS as well as the TPS scoring systems are frequently used to assess anti-PD-1 eligibility. In the KEYNOTE-158 trial, the CPS (p=0.008) and the TPS (p=0.023) were correlated with response to pembrolizumab, but the CPS identified more responders.32 Consequently, with the findings of the KEYNOTE-28 and KEYNOTE-158 trials, a CPS ≥1 is required to give pembrolizumab treatment, with a consistent intra- and interobserver concordance (around 98% in CC).45 IHC for PD-L1 with 22C3 is a companion assay that is now used in many studies to identify patients who may benefit from pembrolizumab. Based on this assay, in the KEYNOTE-158 trial, all patients with CC who responded to pembrolizumab had a CPS ≥1, with no response observed in those with a CPS <1. In contrast, in the phase II study evaluating the anti-PD-1 balstilimab in patients with advanced CC pretreated by platinum-based regimen in the first line, benefits of this ICI were observed regardless of PD-L1 status as assessed by CPS score (ORR 15% in the overall population, 20% in the PD-L1-positive population and 7.9% in the PD-L1-negative population).34 Similarly, the EMPOWER-cervical1/GOG-3016/ENGOT-Cx9 trial demonstrated a benefit in terms of OS in favour of cemiplimab versus investigator choice chemotherapy in patients with PD-L1 expression (detected using the SP263 monoclonal antibody) in tumour cells <1%, although the benefit was larger in patients with PD-L1 expression in tumour cells ≥1.39 Finally, in KEYNOTE-826, 11.2% of patients were PD-L1 negative, CPS <1; in this subgroup, PFS and OS HRs were around 1, at 0.94 (0.52–1.70) to 1.00 (0.53–1.89), respectively.23 Due to the small size of the PD-L1-negative population and the study not being designed to analyse this population, conclusions are not possible and benefits of pembrolizumab in this population remain uncertain.
Another potential biomarker is the tumour mutational burden, which creates neoantigens that are recognized by T cells and activate T-cytotoxic lymphocytes (CTLs).46 High tumour mutational burden (≥10 mut/Mb) was shown to correlate with pembrolizumab efficacy in solid tumours, including CC.47
Tumour-infiltrating lymphocytes may also correlate with response to treatment and have been evaluated in different malignancies including melanoma.48,49 In patients treated with anti-PD-1, serial biopsy samples of tumours were performed and an increase in CD8+ T-cell density was observed in responders but not in those with disease progression.49 However, the overlap in baseline CD8+ T-cell density between responders and those progressing, means that further work in defining a clinically useful cutoff value is still required.
In conclusion, the KEYNOTE-826 study can provide significant insights and directions for ongoing clinical studies, especially in the search for predictive biomarkers, such as PD-L1 status.
Is there a need for bevacizumab or not?
Another unresolved question from the KEYNOTE-826 study is the role of bevacizumab as an adjunct to chemotherapy and pembrolizumab. Prior to the KEYNOTE-826 study, the standard of care was chemotherapy associated with bevacizumab, based on the results of the GOG-240 trial,10,11 especially in terms of OS of this combination: 16.8 months versus 13.3 months with chemotherapy alone (HR, 0.77; 95% CI, 0.62–0.95; p=0.007). However, the toxicity profile that results from the addition of bevacizumab (e.g. fistula in 15%) makes it important to carefully balance the pros and cons of this treatment.11 In this trial, chemotherapy was administered until progression of disease, with a median number of 6 cycles; in the KEYNOTE-826 trial, chemotherapy was prescribed for only 6 cycles. In this latter study, 63% of patients in both arms received bevacizumab. In the subgroups without bevacizumab, HRs were not significant: 0.74 (95% CI, 0.54–1.01) and 0.74 (95% CI, 0.53–1.04) for PFS and OS, respectively.23 This can be interpreted in two ways: chemotherapy and pembrolizumab is not superior to chemotherapy alone or the study was not powered to analyse efficacy in chemotherapy–bevacizumab with or without pembrolizumab. Alternatively, the HR is clearly greater when bevacizumab is also used in combination with chemotherapy, suggesting the importance of combining all drugs together to maximize efficacy. The rationale is strong for the synergistic effect of antiangiogenic drugs and ICIs, and preclinical models showed that inhibition of vascular endothelial growth factor pathway activates an antitumour immunity response, improving the efficacy of ICIs.50–52 The BEATcc trial (NCT03556839)53 will help to answer the question of efficacy for the association of bevacizumab and anti-PD-(L)1. In this phase III trial, patients with advanced metastatic CC are randomized to receive platinum-based chemotherapy and bevacizumab with or without atezolizumab. We await the results, which are expected in March 2023.53
Next steps
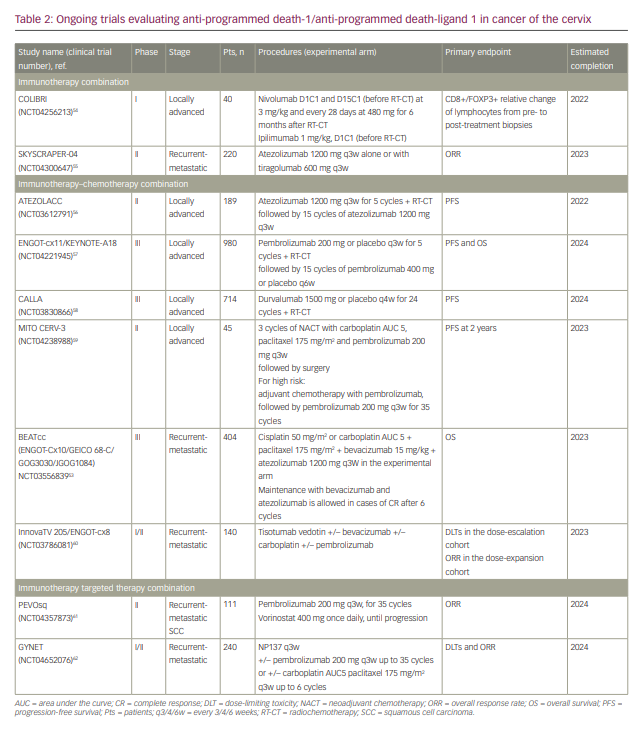
Development of ICIs and other types of immunotherapies in different stages of CC are ongoing (Table 2).53–62
Locally advanced cervical cancer
Several clinical trials are evaluating the combination of ICI with concomitant radiochemotherapy in locally advanced CC (Table 2).54,56–59 The ENGOT-cx11/KEYNOTE-A18 trial (NCT04221945)57 is a randomized phase III study assessing the efficacy of radiochemotherapy with pembrolizumab or placebo in the locally advanced setting. Pembrolizumab at 200 mg every 3 weeks is administered in the experimental arm during radiotherapy and then for 15 additional cycles. In the same design, the open-label ATEZOLACC trial (NCT03612791)56 and the CALLA trial (NCT0383086)58 evaluate the addition of atezolizumab and durvalumab, respectively, to radiochemotherapy. For stage IB2-IIB CC, the single-arm study MITO CERV-3 (NCT04238988)59 assesses the combination of pembrolizumab and carboplatin–paclitaxel doublet in the neoadjuvant setting. Patients can undergo surgery if there is no progression of disease after three cycles of treatment; in patients with high-risk factors of relapse at pathological evaluation, three further cycles of the same combination can be initiated followed by pembrolizumab maintenance for 35 cycles.59
Advanced cervical cancer
After the interesting results of the KEYNOTE-826 study, expectations are focused on the BEATcc trial (ENGOT-Cx10/GEICO 68-C/GOG3030/JGOG1084), a randomized, open-label, phase III study of cisplatin and paclitaxel chemotherapy with bevacizumab with or without atezolizumab as first-line treatment for advanced CC.53 InnovaTV 205/ENGOT-cx8 (NCT03786081)60 is a multi-arm phase I-II clinical trial investigating tisotumab vedotin, a monoclonal antibody-targeting tissue factor conjugated to monomethyl auristatin E, a potent inhibitor of cell division, in monotherapy, and in combination with bevacizumab, pembrolizumab or carboplatin, in the advanced setting. At the interim analysis, the primary endpoint of ORR was 35% in the pembrolizumab–tisotumab vedotin arm in previously pretreated patients.63
Beyond PD-(L)1 blockade new combinations
After interesting results evaluating the efficacy of anti-PD-1 in monotherapy with or without chemotherapy, questions remain about the combination of anticancer immunotherapies, to improve T-cytotoxic lymphocytes response.
Immune checkpoint inhibitor combinations
Double immune checkpoint blockade has an interesting rationale. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a negative regulator of T-cell activation; when CTLA-4 is inhibited, the T-cell response is activated against cancer.64 Similarly to the PD-1 pathway involved in negative T-cell regulation, CTLA-4 inhibition enhances the recovery of T-cell cytotoxicity towards tumours.65 Thus, the human IgG1κ anti-CTLA-4 monoclonal antibody ipilimumab is frequently used in monotherapy or in association with anti-PD-1 as treatment for different solid tumours, such as melanoma and clear cell carcinomas.66 Different early-phase trials combining ICIs, such as nivolumab and ipilimumab (CHECKMATE 358),36 durvalumab and tremelimumab (NCT01975831),37 and balstilimab and zalifrelimab (NCT03495882),38 in advanced CC progressing after at least one line of treatment have demonstrated promising results. In addition, the COLIBRI study (NCT04256213)54 is a multicentre, single-arm pilot study assessing the biological impact pre- and post-treatment with nivolumab and ipilimumab just before concurrent radiochemotherapy in patients with squamous cell CC. In patients with International Federation of Gynecology and Obstetrics stage IB3 to IVA CC, standard first-line therapy is concomitant radiochemotherapy. As described above, nivolumab and ipilimumab have complementary efficacy action in advanced pretreated CC.54 Unfortunately, this combination is explored in heavily pretreated patients with limited immune resources. Patients with diagnosis of local disease might not be immune exhausted and thus might benefit more from this combination. In the COLIBRI trial, patients received nivolumab at 3 mg/kg and ipilimumab at 1 mg/kg before radiochemotherapy, and nivolumab monotherapy at 480 mg every 4 weeks for 6 months after radiochemotherapy.54 The aim is to assess the impact of nivolumab and ipilimumab doublet before radiochemotherapy and of the maintenance therapy on the evolution of the CD8+/FOXP3+ ratio of lymphocytes in primary tumour and on the systemic immune response.54
Beyond anti-CTLA-4/anti-PD-1 doublet, tiragolumab is a novel agent designed to bind to T-cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT). TIGIT is upregulated in most cancer types and its expression is correlated with CTLA-4, PD-1, PD-L1 and tumour mutational burden.67 Therefore, atezolizumab, alone or in combination with tiragolumab, is under investigation in the phase II trial SKYSCRAPER-04 trial (NCT04300647).55
Finally, further early-phase trials are evaluating the combination with vaccines shown to induce HPV16 E6- and E7-specific T-cell responses or with adoptive cell therapy.68–70
Pembrolizumab combined with other therapies
Other strategies are under investigation in early-phase trials. For example, pembrolizumab is being tested in association with vorinostat, a histone deacetylase inhibitor, in patients with advanced squamous cell carcinoma, including a CC cohort (PEVOsq trial, NCT04357873).61 NP137, an anti-netrin-1, is being tested in the first-in-human trial GYNET (NCT04652076).62 By blocking netrin-1, NP137 can restore apoptosis in tumour cells both in vitro and in vivo, leading to efficacy in multiple animal cancer models.71 The aim of the GYNET trial is to evaluate the safety and the clinical efficacy of NP137 combined with pembrolizumab and/or carboplatin–paclitaxel regimen in patients with advanced endometrial and cervical cancers. Finally, there are ongoing early-phase studies that aim to identify different biomarkers of response to immune therapies, beyond anti-PD-(L)1 and ICI.
Conclusion
Despite well-established primary prevention with vaccines and secondary prevention with screening programmes, CC is still one of the most common cancers in women worldwide, with a poor prognosis in advanced settings. As HPV infection provokes immune escape, restoration of T-cell lymphocyte response with ICI, and particularly with anti-PD-(L)1, is an interesting strategy to improve outcomes in patients with advanced CC. Thus, the KEYNOTE-826 study demonstrated clinically significant improvements in PFS and OS with pembrolizumab as an adjunct to chemotherapy, regardless of bevacizumab administration or of PD-L1 status. Pembrolizumab and chemotherapy, associated with bevacizumab in selected patients, should become the new standard of care for women with persistent, recurrent and metastatic CC. These results are promising for the future treatment of this cancer, notably in early settings. Nevertheless, we must not forget to focus on primary prevention, with HPV vaccination being the best immunotherapy and remaining the priority for eradication of this disease in future decades.