Hodgkin’s lymphoma (HL) is a highly curable haematological malignancy with an overall survival (OS) in the early stages of around 90 % with modern first-line therapies.1 In patients being diagnosed in advanced stages, the survival rate for 10-year OS is only above 50 %.2 Despite advances in the treatment of HL, patients having refractory or relapsed (RR) disease still have a poor prognosis.3
Standard Therapy for Relapsed Hodgkin’s Lymphoma
Patients having primary RR disease nowadays remain a clinical challenge. Autologous stem cell transplantation (ASCT) is considered the standard of care for patients having relapsed chemosensitive disease. Two randomised trials showed significant benefit in terms of freedom from treatment failure (FFTF) for ASCT over conventional chemotherapy (CT) in this group of patients.4,5 These trials have resulted in the recommendation of ASCT at the time of first relapse for even the most favourable of patients, though salvage radiotherapy (RT) can offer an effective treatment for selected subsets.
Patients with HL who relapse after an ASCT have a very poor long-term outcome; there is little information about prognostic factors in this setting. A retrospective analysis of the Lymphoma Working Party (LWP) of the European Group for Blood and Marrow Transplantation (EBMT) and Gruppo Italiano Trapianto di Midollo Osseo (GITMO),6 including 511 adult patients having relapsed HL after ASCT, indicated that after a median follow-up of 49 months, these patients’ OS was 32 % at 5 years; independent risk factors for OS were early relapse after ASCT, stage IV disease and bulky disease at the time of relapse, poor performance status and age ≥50 years. For patients having no risk factors, OS at 5 years was 62 % compared with 37 % and 12 % for those having 1 and ≥2 factors, respectively. Relapsed disease after ASCT represents a clear unmet need. Therapeutic options in this subgroup of patients are heterogeneous and include salvage CT or RT (regardless of whether followed by a second SCT), palliative care, new drugs and biological agents. High-dose CT supported by allogeneic SCT (allo-SCT) is a suitable approach for young and fit patients having chemosensitive disease and who have an human leukocyte antigen (HLA)-compatible donor available. A broad spectrum of evidence supports the existence of a potentially beneficial graft versus HL, with those patients developing chronic graft versus host disease (GvHD) presenting with significantly lower relapse rates after transplantation. Patients relapsing more than 3 years after a first ASCT might be suitable for a second ASCT.7 Patients with relapsed disease after an allo-SCT represent a major clinical challenge, with no standard of care.
Brentuximab Vedotin
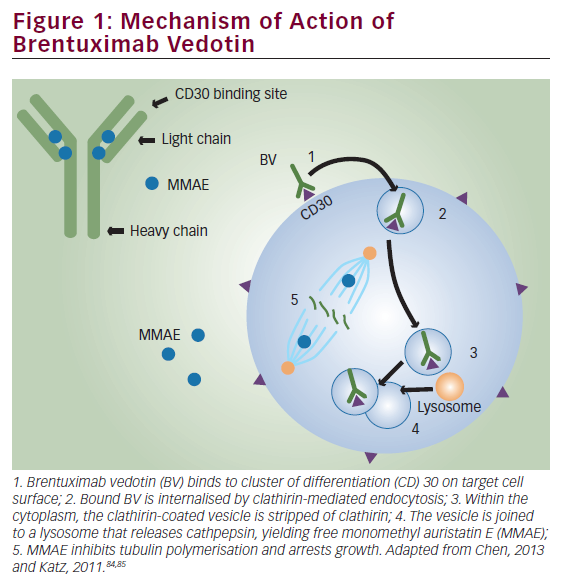
Brentuximab vedotin (BV) (previously known as SNG-35 [Seattle Genetics, WA, US]) is an antibody drug conjugate (ADC) that selectively delivers monomethylauristatin E (MMAE), a very potent antimicrotubule agent, into CD30-expressing cells. Binding MMAE to tubulin disrupts the microtubule network within the cell, subsequently inducing cell-cycle arrest and apoptosis.8 On 19 August 2011, the US Food and Drug Administration (FDA) granted accelerated approval of BV for two indications in patients having HL: patients who fail an ASCT procedure or patients in whom ASCT is not an option after failure of at least two prior multi-agent CT regimens. In addition, BV is also approved in patients having another CD30 positive malignancy, systemic anaplastic large cell lymphoma (sALCL), after failure of at least one prior multi-agent CT protocol.9
Brentuximab Vedotin – Results of Pivotal Phase I/II Prospective Clinical Trials
Two phase I dose-escalation single-arm open-label trials have been published in patients having RR CD30 positive malignancies. The SG035-0001 trial administered BV every 3 weeks,8 whereas SG035-0002 trial administered the drug weekly.10 These trials explored the safety, maximum-tolerated dose (MTD) and dosing the drug once a week and every 3 weeks. Compelling clinical results with a tumour regression percentage of around 85 % were found. The interesting results seen in the phase I trial led to the development of two phase II trials, one of them evaluating the efficacy and safety of BV in patients having RR HL failing an ASCT.11 A total of 102 patients were treated with BV 1.8 mg/ kg by intravenous infusion every 3 weeks. In the absence of disease progression or prohibitive toxicity, patients received a maximum of 16 cycles. The primary endpoint was the overall objective response rate (ORR) determined by an independent radiology review facility – 75 %, with complete remission (CR) in 34 % of patients. The median progression-free survival (PFS) time for all patients was 5.6 months, and the median duration of response for those in CR was 20.5 months. The most common treatment-related adverse events were peripheral sensory neuropathy, nausea, fatigue, neutropenia and diarrhoea.
Brentuximab Vedotin in Real Life – Results of Published Named Patient Programs
Table 1 summarises the clinical findings of the different already-reported Named Patient Programs (NPPs). The German Hodgkin Study Group was the first to publish results, with BV as single agent in 45 patients having RR CD30+ HL, who were treated either in an NPP (n=34) or in the context of a safety study associated with the registration programme of this drug. In these very heavily pretreated patients, an ORR of 60 %, including 22 % CR, was documented. The median duration of response was 8 months, and OS at 12 months was 83 %. The most common side event was low-grade peripheral sensory neuropathy (n=14).12
The single-centre study conducted in the UK-treated 24 patients having RR disease enrolled in an NPP during 2010 to 2011. Patients received a median of 5.5 cycles of BV at a dose of 1.8mg/kg every 3 weeks, with assessment made by positron emission tomography (PET)/computerised omography (CT) after cycles 4 and 8 (PET4, PET8). Overall response rate across all histologies was 67 % (HL 72 %; sALCL 60 %), CR rate was 25 % (HL 17 %; sALCL 60 %), median PFS was 5.1 months and toxicity was mild to moderate in the majority of cases, including sepsis (n=5) and sensory neuropathy (n=3). Six patients proceeded to allo-SCT. Best response was seen after four doses, and the authors concluded that consideration of allo-SCT should be made early and scheduled after the first assessment indicating response.13
The Italian group has reported the largest study published to date, including 65 heavily pretreated patients diagnosed with HL with a median age of 27.5 years. Of them, 88 % had undergone a previous ASCT, 5 % had also failed an allo-SCT and 69 % were refractory to first-line therapy. Median number of cycles of BV was eight, at a dose of 1.8 mg/kg every 3 weeks. Response was assessed by PET-CT scan after cycles 3 and 8 PET3, PET8). ORR at the first restaging was 70.7 %, including 21.5 % of CR, indicating that consolidation should be considered early. ORR after PET8 was 45.5 %, with an OS at 20 months of 73.8 % and a median PFS of 6.8 months.
Peripheral neuropathy was, again, the most common adverse event (21.5 %), though less frequent than in the pivotal phase II study.14 The recently published Turkish experience reported similar results in a group of 58 patients having HL (median age 26) who received a median number of seven cycles of BV. Forty-nine patients had failed a prior SCT (39 ASCT and 10 allo-SCT). ORR was 63 %, with 26 % CR in the early evaluation; on treatment prolongation, the ORR was 32 %, with 22 % CR. OS at 12 months was 71 %, with a median PFS of 7 months. Fourteen patients were subsequently transplanted, but only 36 % were in CR before the transplant. BV was well tolerated, with the most common side effects fatigue (50 %) and neuropathy (31 %).15
Finally, the Asian experience16 includes 22 patients (median age 30) who had previously received a median number of three prior treatments. More than 50 % of them were primary refractory, and almost 80 % of them had failed a prior ASCT. ORR was 72.7 %, with a CR rate of 18.2 % (assessment by PET-CT every one or two cycles); median PFS was 5.7 months. The most common side effect event was neutropenia.
The published results of the NPPs indicate that in real life, efficacy and toxicity are similar to what had already been published in prospective clinical trials. As expected, disease responses are very quick, and a substantial proportion of patients was later on consolidated with a SCT procedure, generally an allo-SCT.
The Role of Stem Cell Transplantation in the Era of Brentuximab Vedotin
ASCT is the standard of care for patients with relapse chemosensitive disease. Not all patients perform equally after an ASCT; several welldescribed clinical factors are associated with a poorer long-term outcome after transplant. More recently, the achievement of a PET negative CR status has been associated with a significantly better outcome after ASCT, independent of the number of treatment lines needed to reach this situation.17 The major clinical concern allo-SCT faces nowadays is not non-relapse mortality (NRM), but rather the high relapse rate that patients experience after the allogeneic procedure. The introduction of BV in these two clinical settings has allowed to significantly modify this landscape.
Brentuximab Vedotin and Autologous Stem Cell Transplantation
BV is able to facilitate patients undergoing an autologous procedure. Sasse et al.18 retrospectively analysed 14 patients having primary RR HL who were treated with BV as single agent in an NPP and who had not received a prior ASCT out of consideration for their refractory disease (n=9), comorbidities (n=4) or unknown factors (n=1). BV resulted in an ORR of 71 % (10/14) with five CR. Five patients having refractory disease and four patients having relevant comorbidity responded. Consolidating ASCT (n=4) or allo-SCT (n=1) was performed in five patients. Median PFS was 9 months, with the median OS not reached. These data indicated the capacity of BV to take into ASCT otherwise chemorefractory patients.
Moskowitz and co-workers19 have tested the capacity of BV single drug per two cycles to achieve a PET-negative CR in patients having relapsed HL to avoid salvage CT before ASCT. Forty-six patients were included in this investigator-initiated strategy (45 were evaluable), all receiving two cycles of BV at the usual schedule. Twelve patients achieved a PETnegative CR after BV and underwent the ASCT procedure. The remaining 30 patients were treated with augmented ifosfamide, carboplatin and etoposide (ICE) for two cycles. Twenty-one patients achieved PETnegative status and were autografted. The eight last patients underwent different strategies, seven ending up in the ASCT programme. In total, 39 of 45 evaluable patients were able to be autografted; event-free survival of the whole series was greater than 80 %.
BV has also been tested in platinum-refractory patients with the objective of increasing the PET negativity rate before transplantation. Fifteen consecutive patients having RR HL and fluorodeoxyglucose (FDG) PET–positive disease after platinum-based salvage therapy were treated with a median of four cycles of BV. Normalisation of FDG PET (Deauville ≤2) occurred in 8/15 (53 %) patients but was only observed in patients who had achieved partial remission or stable disease after platinum-based salvage therapy. All patients eventually proceeded to ASCT, regardless of FDG PET status.20
An additional investigation is being undertaken with BV in the ASCT setting. Two ongoing prospective phase I-II clinical trials are testing the capacity of BV combined with a conventional salvage protocol to increase the ORR and the percentage of patients presenting PETnegative for an ASCT. In addition to that, two ongoing clinical trials (NCT01508312 and NCT01393717) are investigating BV as a single agent as salvage therapy prior to high-dose chemotherapy and ASCT in patients having relapsed HL. Finally, the first presented results coming from the AETHERA trial indicate that BV given shortly after the ASCT at a dose of 1.8 mg/kg every 21 days, up to 16 doses, can decrease the relapse rate after transplantation and significantly improve PFS in patients having HL at high relapse rate after transplant.21
Brentuximab Vedotin and Allogeneic Stem Cell Transplantation
The use of reduced-intensity conditioning (RIC) protocols has allowed a significant reduction on NRM after the allogeneic procedure. The major cause of failure of allo-SCT is disease relapse after transplantation; patients having chemosensitive disease do significantly better than patients having refractory disease. BV is an interesting treatment option for bridging patients into the allogeneic transplantation without significant haematological and extrahaematological toxicity, as opposed to conventional salvage CT, and with less disease burden, owing to the effectiveness demonstrated in the pivotal phase II trial. Chen et al.22 retrospectively examined the records of 18 patients having RR HL who were treated in BV clinical trials to evaluate the efficacy and safety of subsequent RIC-allo. Seventeen patients had previous ASCT, six were in CR and eight were in partial remission before allo-SCT, with 12 grafts from unrelated or mismatched donors. The 1-year OS was 100 %, PFS was 92.3 % and NRM was 0 % (median follow-up 14 months). The incidence of acute GvHD was 27.8 %, and chronic GvHD was 56.3 %. BV before RICallo did not appear to adversely affect engraftment, GvHD or survival and might provide sufficient disease control to enable the allogeneic strategy.
Illidge et al.23 have also reported 15 patients included in the two pivotal phase II studies who received a consolidative allo-SCT following BV treatment. Patients received 1.8 mg/kg BV every 3 weeks for up to 16 cycles. The estimated 2-year PFS rate was 66 %, and the median PFS had not yet been reached. Eleven of the 15 patients were alive, and the estimated 2-year OS rate was 80 %. The safety of BV treatment in this series was consistent with the known safety profile in this setting.
BV may implement the results of allo-SCT by improving disease status at the time of transplant and by allowing patients to enter the allogeneic procedure in a better disease status. Chen et al.24 have also provided an updated report on 21 patients who were treated from 2009 to 2012 with BV before RIC-allo with a uniform fludarabine/melphalan conditioning regimen and donor source after a median follow-up of 29.9 months and have retrospectively compared the patient characteristics and outcomes of these BV-pretreated patients to those of 23 patients who received fludarabine/melphalan RIC-allo without prior BV. Patients who were treated with BV before RIC-allo had a lower median haematopoietic cell transplantation-specific comorbidity index and a reduced number of peri-transplantation toxicities. There were also improvements in 2-year PFS (59 % versus 26 %), mostly thanks to a lower cumulative incidence of relapse/progression (24 % versus 56 %).
HL relapsing after allo-SCT presents a major clinical challenge. BV has been evaluated in 25 HL patients (median age 32; age range 20–56) having recurrent disease after allo-SCT (11 unrelated donors).25 Patients were >100 days after allo-SCT, had no active GvHD and received a median of nine (range 5–19) prior regimens. Nineteen (76 %) had refractory disease immediately before enrollment. Patients received 1.2 or 1.8 mg/kg of brentuximab vedotin IV every 3 weeks (median eight cycles; range 1–16). Overall and complete response rates were 50 % and 38 %, respectively, among 24 evaluable patients. Median time to response was 8.1 weeks, median PFS was 7.8 months and the median OS was not reached. Cough, fatigue and pyrexia (52 % each), nausea and peripheral sensory neuropathy (48 % each) and dyspnoea (40 %) were the most frequent adverse events. The most common adverse events ≥grade 3 were neutropenia (24 %), anaemia (20 %), thrombocytopenia (16 %) and hyperglycaemia (12 %). Cytomegalovirus was detected in five patients (but potentially clinically significant in only one). Both effectiveness and tolerability of BV after allo-SCT seem quite similar to when the drug is administered in autologous transplantation failures.
Finally, BV has been used in combination with donor lymphocyte infusions (DLIs) in patients relapsing after an allo-SCT.26 Four heavily pretreated patients having relapsed HL after allo-SCT a treatment algorithm combining BV infusions (1.8 mg/kg per day every 21 days) with DLI administration in an alternating regimen. Three patients free of GvHD initially thus received DLI in increasing doses that were continued as long as no signs of GvHD greater than I occurred, to a maximum of five doses. Patient 4 had limited chronic cutaneous GvHD before BV treatment and did not receive additional DLI. All four patients showed marked clinical and metabolic responses, with a median duration of disease control of at least 349 days (range 259–366 days) after treatment initiation, which was still ongoing in three patients. Sensory polyneuropathy and mild thrombocytopenia were the most common adverse effects secondary to BV therapy. This study, though focusing on a very small number of patients, demonstrates that BV can induce sustained clinical responses and tumour-specific immunity in an allogeneic setting – warranting additional investigations.
Summary and Future Perspectives
The advent of BV has changed the landscape of RR HL in recent years. BV is a very effective and well-tolerated single drug for treating patients who are not candidates for an ASCT or who relapse after the procedure. In combination with SCT, BV is able to increase the proportion of patients heading into an ASCT, as well as the quality of pretransplant responses. In the allogeneic setting, BV also improves disease status before allo- SCT without increasing end-stage organ toxicity before the procedure. Several questions remain – the capacity of BV to cure patients in the long run, the possibility of increasing ORR before ASCT with the combination of BV and conventional salvage strategies (or, eventually, to avoid salvage CT at all) – at least in a subset of well-responding patients to BV – and the final and mature results of the AETHERA prospective clinical trial.