Hodgkin lymphoma is a B-cell lymphoma that predominantly affects adults between 20–40 years of age, with a second incidence peak in those 55 years of age or older.1,2 It is also the most common form of lymphoma in adolescents, occurring in nearly two-thirds of patients with lymphoma between the ages of 15–19.3 Overall, the annual incidence of Hodgkin lymphoma in the USA and Europe is 2–3 new cases per 100,000, with an annual mortality of approximately 0.4 deaths per 100,000.1,3 In adolescents, the 5-year relative survival rate in patients with Hodgkin lymphoma is 96.4%.3
Hodgkin lymphoma became the first advanced malignancy in adults to be cured by chemotherapy and radiotherapy,4–11 and advances in treatments and patient care over the past 50 years have resulted in ≥85% cure rates in patients with early-stage Hodgkin lymphoma using first-line chemotherapy followed by involved field radiotherapy.4,7–9 However, long-term remission rates in advanced disease do not exceed 70–75% with first-line therapy, and can only be increased with escalated chemotherapy at the expense of increased acute and late toxicity.4,8–11 Furthermore, suboptimal outcomes are observed in those with relapsed/refractory Hodgkin lymphoma.8,9 In these populations, the expected proportions of patients cured using standard therapeutic approaches drops to ~50%.8–11 In addition, there is widespread concern about the acute and long-term toxicity of standard-of-care chemotherapy regimens, particularly for advanced-stage Hodgkin lymphoma and in frail patient populations such as the elderly.8,9 In this review, we summarise the current standard of care for Hodgkin lymphoma, provide an overview of new and developing agents and discuss how these agents may re-shape clinical care strategies.
Current standard of care and unmet needs
Over the past several decades, first-line combined-modality protocols for Hodgkin lymphoma, consisting of chemotherapy and consolidation radiation therapy, have been developed with the dual goal of curing the disease while minimising therapeutic toxicity.8,9 Contemporary clinical research is focused on these same goals.
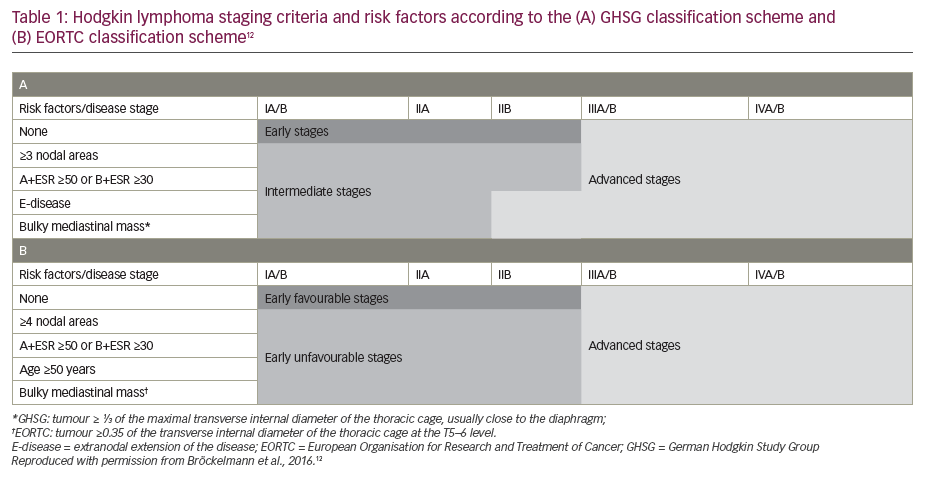
Standard-of-care treatment regimens for patients with Hodgkin lymphoma, as set out in the European Society for Medical Oncology (ESMO) 2018 guidelines, are based largely on a risk classification defined according to the Ann Arbor staging system, combined with the presence or absence of specific prognostic factors.1,2 These include bulky mediastinal mass, albeit evaluated by different methods, the number of affected nodal regions (with a threshold of 3 of 4 nodal areas), and the presence of B-symptoms (fever, night sweats, unexplained weight loss of >10% over 6 months) combined with elevated erythrocyte sedimentation rate, as well as an age of ≥50 years (European Organisation for Research and Treatment of Cancer [EORTC] classification) or presence of contiguous extranodal lesions (German Hodgkin Study Group classification) (Table 1).1,2,12 Another classification system has been developed by the National Comprehensive Cancer Network (NCCN),13 which shows a high sensitivity but low specificity for tumour-specific and survival endpoints, similar to the EORTC and German Hodgkin Study Group classifications.13,14 The ESMO recommendations for the management of Hodgkin lymphoma are as shown in Figure 1 and can be summarised as follows:2
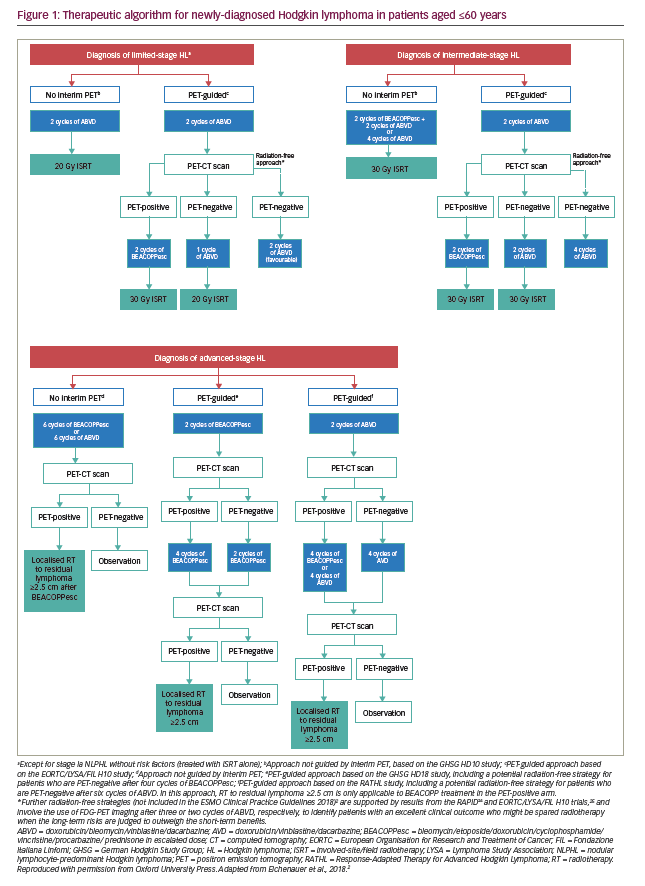
Early-stage disease (or early favourable disease, i.e., no risk factors): Two or three cycles of chemotherapy with doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) followed by involved field radiotherapy (20–30 Gy).
Intermediate-stage disease (or early unfavourable disease, i.e., one or more risk factors): (i) Four cycles of ABVD and involved field radiotherapy, or (ii) a more intensive chemotherapy consisting of two cycles of bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone (BEACOPP-escalated), followed by two cycles of ABVD and supplementary involved field radiotherapy.
Advanced-stage disease (III–IV or even IIB with mediastinal bulk and/or extranodal extension): Six cycles of ABVD or six cycles of escalated BEACOPP, with localised radiotherapy only on residual lymphoma sites as evaluated by fluorodeoxyglucose (FDG) positron-emission tomography/computed tomography (PET/CT). Evidence from several studies suggests that patients with advanced disease who have a positive interim PET (generally defined as a Deauville 5-point scale score of 4 or 5)11 following two cycles of ABVD benefit from switching to an intensified, BEACOPP-based protocol,15–19 or an alternative intensified approach with salvage chemotherapy followed by autologous transplantation, although this strategy is less well established.20 Conversely, after two cycles the pulmonary toxic effects of bleomycin may be avoided in patients with a negative PET/CT, while maintaining efficacy.16 In patients with advanced Hodgkin lymphoma who begin therapy with a BEACOPP-based regimen, interim PET/CT may be able to identify those patients for whom therapy can be de-escalated to ABVD,21,22 or who can receive a reduced number of BEACOPP cycles,23 without negatively affecting treatment outcomes.
Radiation-free approach to further reduce toxicity: This is a potential strategy that was investigated in the RAPID24 and EORTC/LYSA/FIL H10 trials,25 and involves the use of FDG-PET imaging after three or two cycles of ABVD, respectively, to identify patients with an excellent clinical outcome who might be spared radiotherapy, when the long-term risks are judged to outweigh the short-term benefits. Encouraging results have been reported in patients with early unfavourable disease and a negative PET scan after two cycles of ABVD, in whom radiotherapy was omitted in favour of two additional cycles of ABVD, after the completion of four cycles of ABVD.25 However, despite the similar mid-term survival of irradiated and non-irradiated patients, non-inferiority failed to be shown in terms of the primary endpoint (progression-free survival [PFS]) in both these trials as well as the HD16 trial, meaning that reduced toxicity can only be achieved at the cost of reduced tumour control.24–26 The definition of interim PET negativity was strict in all these trials, which generally included only patients with Deauville scores 1 and 2.24–26
Relapsed/refractory Hodgkin lymphoma: High-dose chemotherapy followed by autologous stem cell transplantation (ASCT), a treatment regimen established in the 1990s based on the results of a British National Lymphoma study, and supported by the results of a study by German Hodgkin Lymphoma Study Group and the European Group for Blood and Marrow Transplantation,27,28 is the standard of care in this clinical setting as second-line therapy. Salvage treatments incorporating platinum agents, high-dose cytarabine or gemcitabine such as ifosfamide, carboplatin, and etoposide (ICE), dexamethasone, high-dose cytarabine, cisplatin (DHAP), etoposide-steroid-cytarabine-cisplatin (ESHAP) and ifosfamide, gemcitabine and vinorelbine (IGEV) are used prior to high-dose chemotherapy to eliminate or reduce the tumour burden and to mobilise sufficient stem cells. In addition, consolidation therapy with brentuximab vedotin following high-dose chemotherapy and ASCT, has been shown to significantly improve PFS, but not overall survival, in high-risk patients.29,30
While these treatment strategies have proved successful, there are clear areas for improvement, particularly with regard to the toxicity of therapy, associated short- and long-term complications and patient quality of life. Bleomycin pulmonary toxicity is a well-described complication of bleomycin-containing chemotherapy regimens, such as ABVD, and is responsible for a significantly reduced 5-year overall survival compared with bleomycin-treated patients without bleomycin pulmonary toxicity.31 In elderly patients, even a baseline dosage of BEACOPP is associated with an increased risk of acute toxicity and toxic death, and is therefore not recommended for use in patients over the age of 60.2,32
Although cure rates are respectable in patients with early stage Hodgkin lymphoma (≥85%),4,5,9 approximately 50% of patients with relapsed/refractory Hodgkin lymphoma treated with standard salvage therapies, high-dose chemotherapy and ASCT will experience further relapse.9,10 Moreover, older patients with relapsed Hodgkin lymphoma are often intolerant to salvage regimens and high-dose chemotherapy followed by ASCT.33 Patients with Hodgkin lymphoma who relapse after ASCT have a poor long-term prognosis, with an expected overall survival of 32% after 5 years, which is further reduced to 12% in patients with two or more adverse factors at relapse (<6 months after ASCT, stage IV disease, bulky disease, poor performance status and age ≥50 years).34 Even following successful treatment, survivors of Hodgkin lymphoma are over four times more likely than the general population to develop secondary neoplasms, and the increased risk may persist for over 40 years.35 Hodgkin lymphoma survivors treated in adolescence or adulthood also have a persistent, lifetime increased risk of cardiovascular diseases,36 which may be chiefly driven by radiation; the risk of coronary heart disease in Hodgkin lymphoma survivors has been shown to be directly related to the dose of involved field radiotherapy used, with a 2.5-fold increased risk of coronary heart disease for patients receiving a mean dose of 20 Gy to the heart from mediastinal radiotherapy compared with untreated patients.37
Other long-term consequences of Hodgkin lymphoma treatment include infertility, pulmonary dysfunction, endocrinopathies (e.g. hypothyroidism), muscle atrophy and diabetes.38,39 In addition, many Hodgkin lymphoma survivors experience psychosocial problems that can substantially impact their quality of life, such as impaired memory and concentration, depression and anxiety, sexual dysfunction and unexplained persistent fatigue.39 In particular, a pooled analysis of data from 4,215 patients enrolled in three first-line clinical trials (HD13, HD14, HD15) shows a high incidence of severe acute and persistent fatigue in Hodgkin lymphoma survivors, which is largely independent of tumour stage and treatment, highlighting the need to develop new intervention strategies.40
Overall, despite the high success rate in curing patients with Hodgkin lymphoma, there are clearly still unmet medical needs that require new and effective agents. These include improved therapeutic strategies to maximise efficacy while reducing toxicity, thereby improving long-term patient quality of life and reducing long-term consequences of successful therapy (e.g. secondary cancer, cardiovascular disease, persistent fatigue), as well as improved therapies for older patients, patients with relapsed/refractory Hodgkin lymphoma and those failing ASCT.
New and developing treatments and their potential role in standard of care
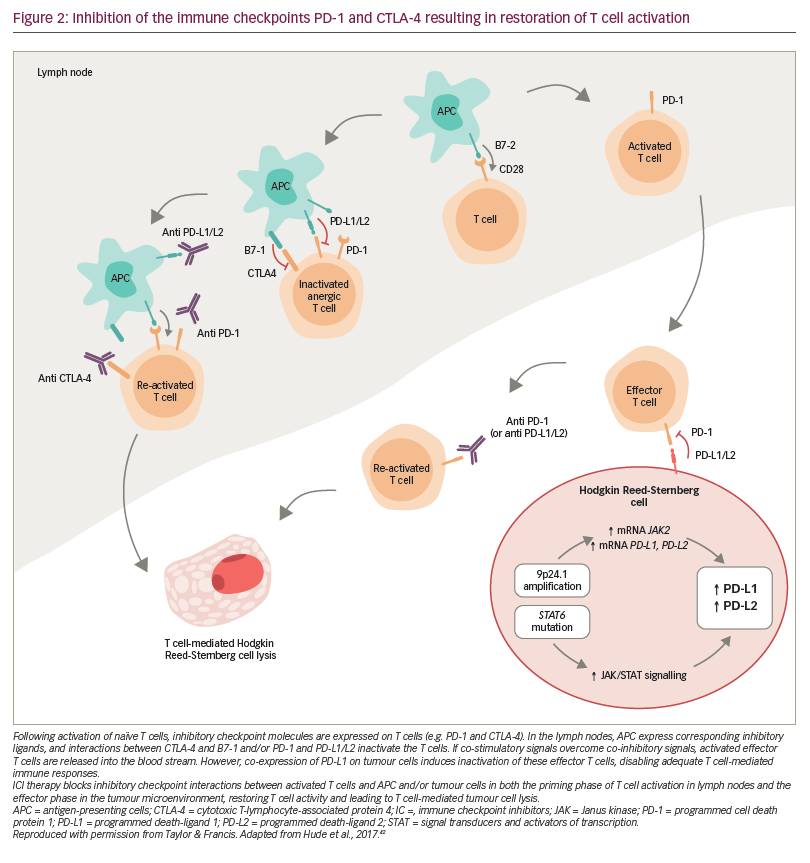
Since the introduction of ABVD chemotherapy in the 1970s, advances in our understanding of the molecular biology of Hodgkin lymphoma have identified a variety of new therapeutic targets, such as lymphoma-specific antigens and immune pathway checkpoints. Lymphoma-specific antigens, like CD30, which is universally present on the surface of the neoplastic cells in classical Hodgkin lymphoma, enable the development of monoclonal antibodies or monoclonal antibody–drug conjugates targeted against the tumour.41 Tumours can evade natural immune responses by overexpressing immune checkpoint receptor ligands, resulting in T cell inactivation.42 The subsequent inhibition of immune pathway checkpoints can therefore restore T cell activation and stimulate an effective T-cell-mediated anti-lymphoma response (Figure 2).42 In Hodgkin lymphoma these include the programmed death (PD) pathway and the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) pathway.42 Part of the PD pathway, PD-1 is an inhibitory receptor expressed on activated T cells, which regulates the effector phase of the immune response by interacting with the ligands PD-L1 and PD-L2. Importantly, these ligands are expressed on many malignant cells and PD-L1/PD-L2 alterations are a defining feature of classical Hodgkin lymphoma.42,43 CTLA-4 is also expressed on activated T cells, and is involved in the priming phase of an immune response.42 It has been shown that treatments directed at both these targets provide an effective anti-lymphoma response with limited toxicity compared with standard-of-care salvage chemotherapy,42 and there is interest in exploring them as part of the first-line treatment strategy for Hodgkin lymphoma as well. These, and other new and developing agents for the treatment of Hodgkin lymphoma, are summarised below and in Table 2.
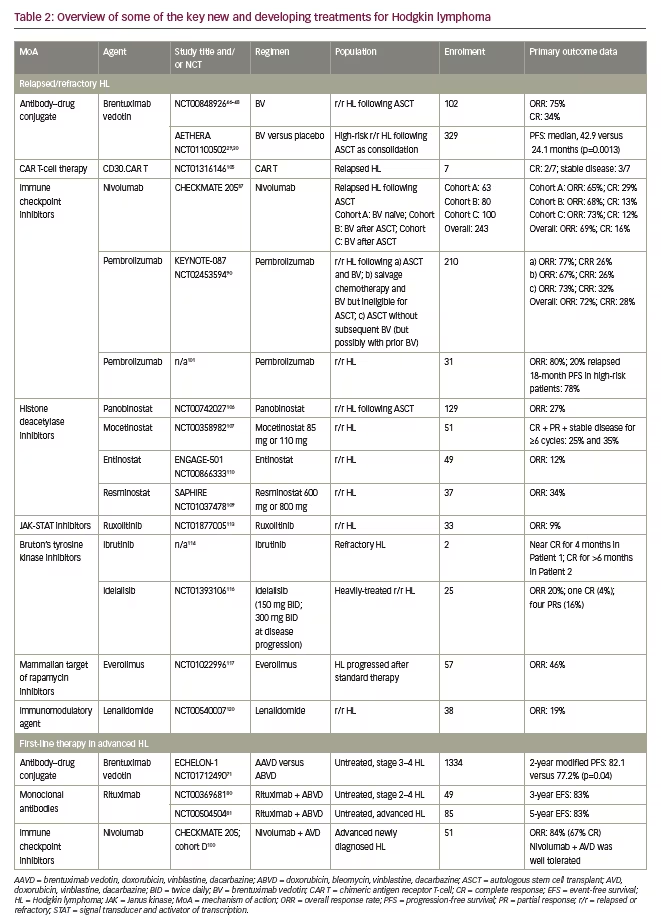
Monoclonal antibodies and antibody–drug conjugates
Brentuximab vedotin
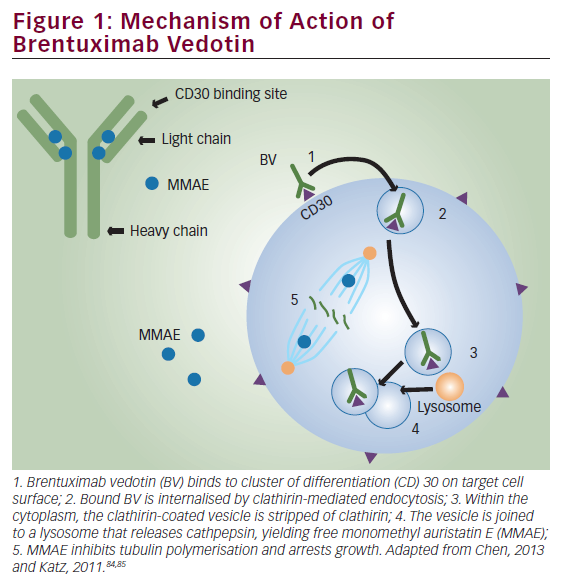
An antibody–drug conjugate, brentuximab vedotin acts primarily by selectively delivering the microtubule toxin monomethylauristatin E into CD30-expressing cells, inducing cell-cycle arrest and apoptosis.44 Brentuximab vedotin has shown promising results in phase I and II clinical trials for the treatment of relapsed/refractory CD30-postive Hodgkin lymphoma.44–47 In the pivotal phase II trial of brentuximab vedotin,46,47 an overall response rate (ORR) of 72% and a complete remission (CR) rate of 33% were reported in 102 patients with relapsed or refractory Hodgkin lymphoma following ASCT failure after a median of 3 years follow-up.48 Similarly, several retrospective cohort analyses in patients with relapsed/refractory Hodgkin lymphoma, mostly those included in named patient programmes, reported ORRs of 60–81% and CR rates of 17–45% with brentuximab vedotin.49–55 Although difficult to assess, recent real-world data suggest that the introduction of brentuximab vedotin in the post-ASCT relapsed/refractory setting may improve overall survival compared with previous standards of care.55,56 Importantly, the pivotal trial demonstrated that a small, but not negligible, minority of patients with relapsed/refractory Hodgkin lymphoma failing ASCT may achieve long-term CR and thus reach potential cure with up to 16 infusions of brentuximab vedotin monotherapy.46–48
Following on from these results, in the phase III AETHERA trial, brentuximab vedotin significantly improved PFS after ASCT compared with placebo (median 42.9 versus 24.1 months; hazard ratio [HR]: 0.57; p=0.013),29 and provided sustained benefits on PFS for at least 5 years (PFS at 5 years: 59% versus 41%; HR: 0.52 [95% CI 0.38–0.72]).30 While there was no overall survival benefit observed in the interim analyses of the AETHERA data, this was chiefly attributed to the small number of events and confounding due to the very high crossover rate permitted in the placebo arm.29 Overall, 85% of patients in the placebo arm received brentuximab vedotin therapy following disease progression, compared with 18% in the brentuximab vedotin group.29 Nevertheless, the evidence suggests that brentuximab vedotin may reduce the need for subsequent toxic treatments.29 Based on the results of AETHERA, brentuximab vedotin was approved in the USA and the EU as the sole consolidation strategy following ASCT in adult patients deemed at high-risk of relapse or progression.57,58
The effectiveness and tolerability of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma following ASCT naturally led to the evaluation of brentuximab vedotin as second-line, first-salvage therapy prior to ASCT in patients with relapsed or primary refractory Hodgkin lymphoma.59 Phase I and II studies of brentuximab vedotin given as single-agent or in combination with chemotherapy either sequentially or concurrently (with augmented ICE/ICE, DHAP, ESHAP), reported ORRs of 68–75% and CRs of 35–50% for single agent therapy,60–62 as well as ORRs ranging from 87–100% and CRs ranging from 70–100% with combination therapy.59,63–67 In addition, several recent studies have evaluated the use of brentuximab vedotin as first-salvage therapy for relapsed or refractory Hodgkin lymphoma in combination with bendamustine (as a potentially less toxic alternative to platinum- or gemcitabine-based chemotherapy), and with nivolumab in an almost chemotherapy-free approach.68–70 In combination with bendamustine, LaCasce et al. reported a metabolic ORR of 92% and a CR rate of 74%.68 Following ASCT the estimated 2-year PFS was 63% and OS was 94%.68 In more heavily pre-treated patients, O’Connor et al. reported an ORR of 78%, CR rate of 43%, 2-year PFS of 60% and 2-year OS of 80% with brentuximab vedotin–bendamustine combination therapy.69 Brentuximab vedotin in combination with nivolumab also showed promising results, with an ORR of 82% and CR rate of 61%.70 Overall, these data support the use of brentuximab vedotin as part of first-salvage therapy strategies in patients with relapsed or refractory Hodgkin lymphoma.
More recently, the phase III randomised ECHELON-1 trial showed that brentuximab vedotin plus doxorubicin, vinblastine and dacarbazine (A+AVD) resulted in superior modified PFS compared with ABVD for the frontline treatment of advanced-stage Hodgkin lymphoma (defined as stage III/IV).71 Patients in the A+AVD arm had a 4.9% lower combined risk of progression, death, or non-complete response followed by additional anti-lymphoma treatment compared with ABVD at 2 years (82.1% versus 77.2%, respectively; HR: 0.77; p=0.04).71 Pre-specified subgroup analysis of modified PFS in ECHELON-1 revealed an HR <1 for the A+AVD regimen versus the ABVD regimen in the subgroups of patients from North America, involvement of >1 extranodal site, International Prognostic Score 4–7, men, stage IV disease and patients younger than 60 years of age.71 Although a statistically significant overall survival difference was not demonstrable in the whole population at the time of ECHELON-1 publication,71 this was an interim analysis of overall survival data (A+AVD versus ABVD: HR: 0.73; 95% confidence interval [CI] 0.45–1.18; p=0.20).71 Further, subsequent subgroup analyses suggest an overall survival benefit in patients with stage IV or multiple extranodal sites.72 As A+AVD was associated with a higher rate of febrile neutropenia compared to ABVD, primary granulocyte colony-stimulating factor prophylaxis is recommended with A+AVD therapy in order to reduce the risk of neutropenic complications.71
Based on the results of ECHELON-1, brentuximab vedotin has been approved by the US Food and Drug Administration as A+AVD for the first-line treatment of advanced classical Hodgkin lymphoma (stage III or IV),57 and by the European Medicines Agency for the first-line treatment of CD30-positive stage IV Hodgkin lymphoma.58 However, the role of brentuximab vedotin in the first-line treatment of Hodgkin lymphoma may not be limited to A+AVD; a study by the German Hodgkin Lymphoma Study Group investigated the incorporation of brentuximab vedotin into a BEACOPP-escalated backbone, with concomitant omission of both bleomycin and vincristine (BRECAPP), and then substitution of procarbazine and prednisone with dacarbazine and a 4-day dexamethasone course (BRECADD), which eliminates steroid therapy from the period of severe neutropenia.73 In this randomised phase II study, BRECADD was shown to be a potent new regimen with a more acceptable toxicity profile compared with BRECAPP.73 Following this, BRECADD was selected for further investigation and is now being compared with a standard BEACOPP-escalated regimen in the ongoing HD21 trial (German Hodgkin Study Group; ClinicalTrials.gov identifier: NCT02661503), with the hope that it may emerge as a new standard of care in the future.
A number of further trials of brentuximab vedotin are currently planned or ongoing, including: the BASALT trial (NCT03474133), investigating the use of prolonged brentuximab vedotin as an alternative to ASCT in patients with refractory or relapsed Hodgkin lymphoma; the HALO trial (NCT02467946), investigating brentuximab vedotin administered in combination with bendamustine in elderly patients with previously untreated advanced Hodgkin lymphoma;74 and NYMC-568 (NCT02398240), investigating brentuximab vedotin administered in combination with chemotherapy (doxorubicin, vincristine and/or rituximab) in children, adolescents and young adults with all stages of newly diagnosed Hodgkin lymphoma. Brentuximab vedotin is also being investigated in combination with nivolumab or ipilimumab for the treatment of relapsed or refractory Hodgkin lymphoma (NCT02572167 and NCT01896999, respectively) with promising early results,70,75 and as first-line therapy in older patients (≥60 years of age) with Hodgkin lymphoma (NCT02758717).
In summary, brentuximab vedotin is currently indicated in Europe as A+AVD for the first-line treatment of adult patients with CD30-positive stage IV Hodgkin lymphoma, the treatment of adult patients with relapsed/refractory CD30-positive Hodgkin lymphoma following ASCT or at least two prior therapies if ASCT or multiagent chemotherapy is not an option, and for the treatment of adult patients with CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following ASCT.58 In the USA, brentuximab vedotin is also indicated for use in adults with previously untreated stage III/IV classical Hodgkin lymphoma in combination with AVD.57 However, in addition to its use to consolidate treatment response following ASCT, the results of ECHELON-1 (and potentially HD21) indicate that brentuximab vedotin will likely become an option for the first-line treatment for advanced Hodgkin lymphoma as an adjunct to chemotherapy. In particular, sequential brentuximab–AVD therapy has been shown to be well tolerated and associated with robust outcomes in older patients (≥60 years; ORR: 95%; CR: 90%), with geriatric-based measures strongly associated with patient survival.76 Further, brentuximab vedotin (as monotherapy or in combination with dacarbazine) may also provide an alternative first-line treatment option for older patients who cannot tolerate conventional combination chemotherapy, albeit with inferior results (ORR: 92%; CR: 73% and ORR: 100%; CR: 62% for monotherapy and dacarbazine combination therapy, respectively).77,78
Further evidence on the use of these therapies in specific populations, as well as on the effectiveness of brentuximab vedotin in combination with other agents, may also lead to their integration into limited- or intermediate-stage treatment modalities.
Rituximab
Rituximab is a cytolytic monoclonal antibody that selectively targets the CD20 antigen expressed in many B-cell non-Hodgkin lymphomas, and has broad indications in B-cell non-Hodgkin lymphomas and chronic lymphocytic leukaemia.79 In classical Hodgkin lymphoma, studies investigating the use of rituximab in combination with ABVD (R-ABVD) reported a 5-year event-free survival of 83% and overall survival rates of 96–98%.80,81 However, despite these promising results the R-ABVD regimen was not further developed. In addition, rituximab proved unable to improve patient outcomes in interim PET-positive patients when combined with BEACOPP in advanced Hodgkin lymphoma.19,23,82
Immune checkpoint inhibitors
The mode of action of immune checkpoint inhibitors involves the re-activation of T cells to elicit an anti-tumour response. As such, these agents are not associated with the toxicities of conventional cytotoxic therapy, though they can be associated with autoimmune side effects affecting virtually all organs.83 These immune-related adverse events include skin rash, diarrhoea, pneumonitis, and endocrine toxicities. As haematological toxicities associated with checkpoint inhibitors are rarely dose-limiting, these agents may be suitable candidates for combination therapies.84
Nivolumab and pembrolizumab are IgG4 monoclonal antibodies (human and humanised, respectively) that target PD-1 and inhibit the PD-1 pathway.42 Both nivolumab and pembrolizumab have shown single-agent efficacy in the treatment of relapsed/refractory Hodgkin lymphoma.10,85 In the CHECKMATE 205 study, an ORR of 66.3% was reported after treatment with nivolumab in patients with classical Hodgkin lymphoma who had relapsed or progressed following ASCT and brentuximab vedotin. A CR was observed in 8.8% of patients.86 Extended follow-up of the study population found a median duration of response of 16.6 months and median PFS of 14.7 months, with a favourable safety profile.87 Likewise, for pembrolizumab, the phase Ib KEYNOTE-013 study included patients with relapsed or refractory Hodgkin lymphoma whose disease progressed on or after treatment with brentuximab vedotin. Treatment with pembrolizumab resulted in an ORR of 65%, including 16% of patients who achieved a CR.88
A subsequent phase II study, KEYNOTE-087, showed similar results (ORR of 69.0% and CR rate of 22.4%) in patients who had relapsed or progressed either following ASCT and brentuximab vedotin, or following salvage chemotherapy and brentuximab vedotin (i.e., ineligible for ASCT) or following ASCT without subsequent (but potentially with prior) brentuximab vedotin.89 Although longer follow-up data are warranted, this class of drugs appears to provide prolonged clinical benefit even in a sizeable subgroup of patients who experience disease persistence by current definitions, but remain in a clinical condition justifying the continuation of checkpoint inhibition therapy. For example, the effectiveness of ‘treatment beyond progression’, a rather new concept in the treatment of Hodgkin lymphoma, was recently established in the setting of nivolumab therapy.87
Durable responses were observed with continued treatment beyond progression (61% of patients had stable or further reduced target tumour burdens with a median of 5.2 months additional treatment),87 and the 2- to 3-year overall survival in both the CHECKMATE 205 and KEYNOTE-087 trials exceeded 80%, approaching 90% in specific patient cohorts.87,90 Given that the majority of these patients have failed both ASCT and brentuximab vedotin, this compares very favourably with the survival rates observed after ASCT failure or brentuximab vedotin failure,91 even considering good performance status selection bias.
As a result of these findings, nivolumab is now indicated as monotherapy in adult patients with classical Hodgkin lymphoma that relapsed or progressed following ASCT and treatment with brentuximab vedotin (and in the USA, following three more lines of systemic therapy that includes ASCT), following accelerated approval in the USA.92,93 Pembrolizumab is now indicated as monotherapy for the treatment of adult patients with relapsed or refractory classical Hodgkin lymphoma who have failed ASCT and brentuximab vedotin, or who are transplant-ineligible and have failed brentuximab vedotin in the EU (or in the USA, in adult and paediatric patients with refractory classical Hodgkin lymphoma or those who have relapsed after three or more prior lines of therapy), following accelerated approval in the USA.94,95
Pembrolizumab is currently undergoing a direct comparison with brentuximab vedotin in the phase III randomised KEYNOTE-204 trial (NCT02684292) in patients with relapsed or refractory Hodgkin lymphoma.96 Pembrolizumab is under investigation as combination therapy with the bispecific anti-CD30/CD16A antibody AFM13 (NCT02665650), the immunomodulatory agent lenalidomide in patients with relapsed Hodgkin lymphoma (NCT02875067), the histone deacetylase (HDAC) inhibitors vorinostat (NCT03150329) and entinostat (NCT03179930) in patients with relapsed/refractory Hodgkin lymphoma. A potential synergy between anti-PD-1 agents and the T-cell boosting activity of decitabine has also been revealed in a phase II study of the anti-PD-1 agent camrelizumab in patients with relapsed or refractory Hodgkin lymphoma.97
Beyond the relapsed/refractory setting, nivolumab and pembrolizumab may also have a role as first-line agents in combination with chemotherapy; studies are currently ongoing to assess the effects of AVD and checkpoint inhibitors as first-line therapy for higher risk patients with classical Hodgkin lymphoma (NCT03033914).98–100 Pembrolizumab is also being evaluated as consolidation after ASCT.101 In addition, ipilimumab, a fully human monoclonal IgG1k antibody against CTLA-4, which is currently indicated for advanced melanoma as monotherapy or in combination with nivolumab,102 is also under investigation in Hodgkin lymphoma as combination therapy with brentuximab vedotin with promising early results (phase I study [n=12]: ORR of 67% and CR of 42%).75
Combination therapy of checkpoint inhibitors with brentuximab vedotin may potentially provide an option for chemotherapy-free treatment in relapsed/refractory Hodgkin lymphoma. Long-term results of ongoing trials of nivolumab/brentuximab vedotin, and ipilimumab/brentuximab vedotin are awaited with interest.70,75
Chimeric antigen receptor T-cell therapy
Modified chimeric antigen receptor (CAR) T-cell therapy involves the extraction, modification and reintroduction of T cells from a patient to specifically target an antigen on neoplastic cells. CAR T cells directed against the B-lymphocyte antigen CD19, which is almost universally expressed in B-cell lymphomas as a pan-B cell marker, are now commercially available and have been applied with promising results in the treatment of refractory large B-cell lymphoma and B-cell acute lymphoblastic leukaemia.103,104 Preliminary investigations into the use of CD30-specific CAR T cells in refractory or relapsed Hodgkin lymphoma, while still at a very early stage, are also encouraging: in seven patients with relapsed Hodgkin lymphoma included in a dose-escalation study, clinical responses were observed in two patients (one CR and one continued CR) and stable disease in three patients.105 However, CAR T-cell therapy is a complex and expensive procedure requiring an adequate infrastructure, and potential toxicity concerns may also limit its use.
Additional targeted therapies
Various other targeted therapies are under investigation in Hodgkin lymphoma and have shown promise in early-phase studies, either as single-agent or combination therapies.
Histone deacetylase inhibitors
HDACs, enzymes that remove acetyl groups from lysine residues on histone proteins, have a role in the regulation of oncogenic pathways relating to cell-cycle progression, survival, angiogenesis, and immunity,106,107 making them potential targets for cancer therapies.107 Panobinostat is an HDAC that has been shown to provide favourable clinical activity in heavily pre-treated patients with relapsed/refractory Hodgkin lymphoma, yielding tumour reduction in 74% of patients and an ORR of 27%.106 A randomised prospective trial of panobinostat as consolidation therapy after ASCT for high-risk Hodgkin lymphoma patients was terminated prematurely due to slow recruitment. However, patients already enrolled to the panobinostat arm were provided the drug in an open-label treatment phase and evaluated for safety; panobinostat was judged by the investigators to have an acceptable safety profile in an Hodgkin lymphoma population at high risk of relapse.108
Another HDAC inhibitor, mocetinostat, has also demonstrated potent anti-proliferative effects against Hodgkin lymphoma. In relapsed or refractory classical Hodgkin lymphoma, 81% of patients who completed at least two cycles of mocetinostat therapy (110 mg and 85 mg doses) showed a decrease in tumour measurements with an ORR of 35% and 21% for the two dosage groups, respectively.107 Similarly, a study of the HDAC inhibitor resminostat (600 mg or 800 mg administered for 5 days of a 14-day treatment cycle) demonstrated efficacy, target engagement and epigenetic modulations in heavily pre-treated patients with relapsed or refractory Hodgkin lymphoma, with an ORR of 34%, disease control in 54% and reduced tumour size in 69% of patients.109
Lastly, the HDAC inhibitor entinostat has a greater half-life than other agents in its class, which enables dosing every 1–2 weeks and may potentially improve tolerability. In support of this, the ENGAGE-501 study of entinostat in relapsed and refractory Hodgkin lymphoma showed an ORR of 12%, with 58% of evaluable patients experiencing a reduction in tumour size and the treatment was reported to be well tolerated.110 In addition to their use as a monotherapy, HDAC inhibitors have favourable immune modulatory effects, making them potential candidates for combination therapies with chemotherapy, small molecule inhibitors or immune therapy agents, as described previously.
JAK-STAT pathway inhibitors
The Janus kinase (JAK) family of intracellular non-receptor tyrosine kinases are activated by the binding of cytokines to their associated receptors.111 This triggers recruitment and phosphorylation of signal transducers and activators of transcription (STAT) proteins, which dimerise, relocate to the nucleus, and activate transcription of a series of target genes involved in processes such as cell proliferation and immunity.111 Abnormal activation of the JAK–STAT pathway has been linked to lymphoma, and overexpression of JAK2, in particular, is common in classical Hodgkin lymphoma.111 Ruxolitinib, an agent approved for idiopathic myelofibrosis,112 is a selective inhibitor of the tyrosine kinases JAK1 and JAK2 that is under investigation for the treatment of relapsed/refractory Hodgkin lymphoma. In a phase II study, patients with a median of five lines of prior treatment given ruxolitinib achieved an ORR of 9.4% and a median OS of 27.1 months.113 While the study failed to fulfil efficacy criteria for further development of ruxolitinib as a monotherapy, the favourable safety profile suggests that it may play a useful part in combination therapies in the future.113
Bruton’s tyrosine kinase inhibitors
Bruton’s tyrosine kinase (BTK) plays an early role in the B-cell receptor signalling cascade, which is important for B-cell proliferation and survival.111 Ibrutinib is an irreversible small-molecule BTK inhibitor, which inhibits B-cell receptor signalling by occupying the active site of BTK.111 Although ibrutinib has shown anti-tumour activity in the treatment of other B-cell neoplasms such as mantle cell lymphoma and chronic lymphocytic leukaemia, evidence for ibrutinib in Hodgkin lymphoma is limited, but the results of case studies in patients with primary refractory classic Hodgkin lymphoma suggest that further investigation is warranted.114
Phosphatidylinositol 3-kinase inhibitors and mammalian target of rapamycin inhibitors
The phosphatidylinositol 3-kinase(PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is a key regulator of cell growth, proliferation, survival, metabolism, and angiogenesis, and is often dysregulated in Hodgkin lymphoma.115 Idelalisib is a selective inhibitor of PI3K-δ inhibitor that has shown clinical efficacy in a phase II study of patients with heavily-treated relapsed/refractory Hodgkin lymphoma who had previously received a median of five therapies, including ASCT, brentuximab vedotin, and radiation therapy.116 The ORR was 20%, with a median time to response of 2.0 months, one CR (4%) and four partial responses (16%).116 However, there was an increased incidence of gastrointestinal side-effects such as diarrhoea/colitis (12%) in these patients.116
It is hoped that the next generation of PI3K inhibitors may improve tolerability via different formulations and modes of administration, and enable wider use of these agents. Everolimus is an oral mTOR inhibitor that has demonstrated favourable results in a phase II study of patients with heavily pre-treated, relapsed, or refractory classic Hodgkin lymphoma, in which the ORR was 45.6%, 8.8% of patients achieving a CR, and 12% of patients achieved a long-term response of over 1 year.117 A retrospective report from a named patient programme in Brazil reported a similar ORR of 45.4%, with CR reported in 6% of patients, and three patients adhering to therapy for over 4 years.118 Further investigation may enable the identification of a subgroup of patients who respond particularly well to PI3K and mTOR inhibition, permitting optimisation of the use of these agents.118,119
Immunomodulatory agents
The immunomodulatory agent lenalidomide has also been shown to exhibit anti-tumour activity in patients with relapsed or refractory Hodgkin lymphoma (ORR of 19%),120 which led to its investigation as part of combination therapies with other agents. In a phase I/II study of lenalidomide in combination with bendamustine in patients with chemorefractory Hodgkin lymphoma (NCT01412307), an ORR of 75% (95% CI 59–88) and a CR of 44% was achieved.121 A phase II study of lenalidomide in combination with temsirolimus has also shown promising results in patients with relapsed or refractory Hodgkin lymphoma (ORR of 80%).122 Investigations of lenalidomide in combination with other agents, such as pembrolizumab (NCT02875067) and nivolumab (NCT03015896), are currently underway.
The integration of new agents into treatment combinations, either to replace or intensify current treatment regimens, is the primary goal of research in the treatment of Hodgkin lymphoma. The emergence of targeted therapies and immunotherapies provides new and exciting treatment options. Many of these new agents possess complementary modes of action and have demonstrated synergistic effects with non-overlapping toxicity in small early-phase studies, such as brentuximab vedotin plus ipilimumab or nivolumab. Of these, only brentuximab vedotin, approved as a single-agent therapy in relapsed or refractory Hodgkin lymphoma post-ASCT, currently has robust and mature phase III clinical data in Hodgkin lymphoma.58
Nivolumab is also indicated as monotherapy in adult patients with classical Hodgkin lymphoma that has relapsed or progressed following autologous hematopoietic stem cell transplantation (HSCT) and treatment with brentuximab vedotin (or three more lines of systemic therapy that includes autologous HSCT).92,93 While pembrolizumab is indicated as monotherapy in adult patients with refractory classical Hodgkin lymphoma who have failed, or are ineligible for, ASCT and brentuximab vedotin in the EU, or in adult and paediatric patients who have relapsed after three or more prior lines of therapy in the USA, following accelerated approval.92,94
As more research is conducted, it is hoped that both single-agent and combination strategies will re-shape the therapeutic landscape in Hodgkin lymphoma in several ways. First, by replacing weaker elements of standard chemotherapy with the aim of reducing toxicity and long-term complications in low-risk patients; second, by integrating into standard-of-care therapies for advanced or high-risk patients to improve efficacy and reduce the high toxicity of intensive chemotherapy; and finally, by integrating into standard-of-care therapies for relapsed patients to improve the success of ASCT and/or improve disease control post-ASCT.
Based on current data, it appears reasonable to predict brentuximab vedotin will form part of the first-line therapy for advanced Hodgkin lymphoma. Dependent on the results of future research, combinations of targeted agents including antibody–drug conjugates and immune checkpoint inhibitors may even provide a cytotoxic chemotherapy-free option for the first-line treatment of Hodgkin lymphoma. 
At the EHA conference in Amsterdam we are joined by Professor Theodoros P Vassilakopoulos to discuss the most exciting new developments in standards of care for Hodgkin lymphoma.
Questions
1. What are the limitations of current standards of care for Hodgkin lymphoma (HL)? (0:05)
2. What is the role of brentuximab vedotin in the current treatment paradigm for HL? (2:10)
3. How is the role of brentuximab vedotin likely to further evolve in the treatment of HL? (3:33)
4. How can immune checkpoint inhibitors best fit into the treatment paradigm for HL? (4:45)
5. What emerging therapeutic options seem most promising in the future treatment of HL? (5.30)
Filmed at the European Hematology Association (EHA) 24th congress 2019, Amsterdam, Netherlands.
touchONCOLOGY.com is an independent information resource supporting physicians, clinicians and leading industry professionals in continuously developing their knowledge, effectiveness and productivity, via free-to-access content in multimedia formats.