Chemoimmunotherapy with Rituximab as an Initial Cytoreductive Regimen
Chemoimmunotherapy is a widely accepted first-line approach as rituximab increases response rate (RR) and prolongs PFS. In a German lymphoma study group (LSG) randomised comparison of CHOP with CHOP-R (with rituximab), CHOP-R increased RR from 72 to 92%, CR from 9 to 32% and PFS from 14 to 21 months, although still without a clear OS benefit.4 Although the addition of rituximab to the fludarabine, cyclophosphamide, mitoxantrone regimen (FCM-R) was superior to FCM in relapsed patients, most of the responses were shorter than 12 months.5 A standard-dose chemoimmunotherapy is just not enough to cure MCL, and some kind of post-induction or consolidation treatment is necessary. For maximum effectiveness, whatever we offer to our patients should be incorporated into the first-line approach, before the resistance occurs. Most of MCL patients are either ‘young’ elderly patients or ‘elderly’ elderly patients. In the first group, an intensive therapy approach with subsequent transplant procedure is the treatment of choice.
Dose Escalation for ‘Fit’ Patients
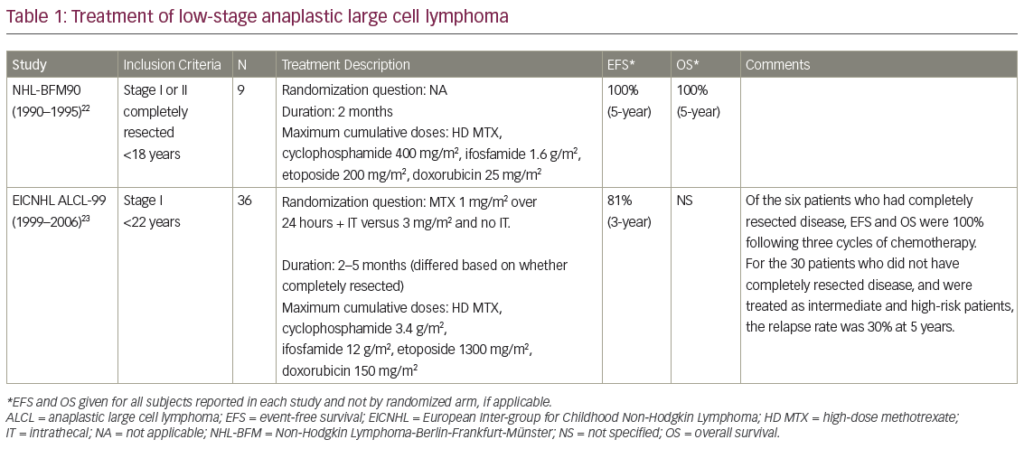
Autologous transplants as consolidation of the first-line therapy became a gold standard after the first generation of European MCL Network trials demonstrated a PFS benefit compared with INF maintenance (medium PFS 39 months versus 17 months, p=0.01).6 In the ongoing secondgeneration trial, the role of the more intensive induction therapy is investigated, comparing CHOP-R with alternative CHOP-R/DHAP-R. High efficiency (PFS >70% at three years) of chemoimmunotherapy regimens containing intermediate-dose cytarabine was shown in phase II studies: alternative HyperCVAD-R/MA-R regimen developed in MD Anderson,7 alternative MaxiCHOP-R/cytarabine-R followed by BEAM conditioned transplant investigated by Nordic MCL Group8 and Milano high-dose sequential chemotherapy.9 The results of the Nordic and Italian groups are particularly interesting, as they were both performed in a multicentre setting and there is a plateau of OS curves after the third year. Present Nordic Group protocol includes radioimmunotherapy (Z-BEAM) in a transplant-conditioning regimen for partial responders. Radioimmonotherapy Consolidation for ‘Elderly’ Elderly Patients?
The majority of MCL patients are not eligible for intensive therapy. In the ongoing second-generation European MCL Network trial, FC-R is compared with CHOP-R. Rituximab increases the response rate to literally all chemotherapy regimens, but the question of post-induction therapy to increase the quality of remission remains open. Data on rituximab maintenance in follicular lymphoma remain preliminary.10 Involved field radiotherapy (IFRT) is effective in a localised disease, as demonstrated in a retrospective analysis from Vancouver (71% versus 25% OS at six years, p=0.01).11 Until recently, total body irradiation (TBI), one of the transplant-conditioning regimens) was the only alternative at the advanced clinical stage. Radioimmunotherapy (RIT) is an efficient method of ‘total tumour radiotherapy’, and is feasible for elderly patients.
Ibritumomab tiuxetan (Zevalin) is a 90Y labelled radioimmunoconjugate binding to the CD20 antigen present on the surface of B lymphocytes. RIT combines the specificity of monoclonal antibodies (MoAb) with the efficiency of radiotherapy. Ibritumomab is administered intravenously and, after a short distribution phase, most of the compound is bound in the tumour. Monoclonal antibodies are relatively big molecules, poorly penetrating enlarged, partly fibrosed lymph nodes and tissue lymphoma infiltrates. A single ibritumomab-tiuxetan molecule, with thousands of 90Y nuclides attached, is capable of killing several adjacent cells within the range of beta particles emitted by 90Y (about 5mm in the tissues). Compared with rituximab, ibritumomab is less selective: what is a desired effect in lymph nodes is a disadvantage in bone marrow. Haematological toxicity is a dose-limiting factor:12 radioimmunotherapy is contraindicated in patients with an important bone marrow lymphoma infiltration (>25%), BM hypoplasia or peripheral cytopaenia (leukocytes <1000/ul, blood platelets <100,000/ul). Other side effects are rare and the therapy is exceptionally well tolerated in terms of quality of life.
Paradoxically, an antilymphoma effect of RIT may be greater in patients with a measurable disease than in micrometastasis or minimal residual disease. In very small tumours, with fewer antibodies attached, a crossfire effect is less pronounced. On the other hand, one should be realistic about the tumour burden that could be effectively treated with a single infusion of ibritumomab (about 2mg). In follicular lymphoma, complete responses were less frequent if lymph nodes were more than 5–7cm in diameter. Large asynchronic tumour masses (i.e. massive splenomegaly) may ‘trap’ most of the infused antibodies (known as the ‘sink phenomenon’), decreasing the treatment’s efficiency.
Summary of Clinical Trials with Radioimmunotherapy in Mantle Cell Lynphoma
The first attempts to use radioimmunotherapy in MCL were in relapsed/refractory cases (see Table 1). In MD Anderson, 22 patients, after failing from one to six previous therapy lines, were treated with ibritumomab.13 Twenty-one out of 22 had previously received rituximab, five out of 22 bortezomib, and 14 out of 22 had relapsed after the HyperCVAD/MA regimen. None of the eight responders had bulky diseases, and the largest measurable lesion did not exceed 3cm. Similar results were confirmed by German Lymphoma Study Group (n=14, 33% RR, medium PFS 3.9 months).14 The length of response would probably increase if patients were treated earlier within the disease course, after initial de-bulking chemoimmunotherapy. This consolidation approach was investigated by the Polish Lymphoma Study Group (PLRG), where 10 patients in two to five relapse were subjected to RIT after three to six cycles of FCM-R.15 The response rate was high (RR = 9/10, CR = 4/10) with the medium PFS 7.5 months (see Figure 1). Haematological toxicity was more pronounced than in the MD Anderson and GLSG studies, with eight out of 10 patients developing grade 3–4 leucopaenia and thrombocytopaenia. Although initial chemotherapy may increase the PFS from 3.9 to 7.5 months, most patients subsequently relapse and ibritumomab does not seem to overcome the disease resistance once it occurs.
There are two multicentre studies, published in ASH and ASCO abstracts, investigating RIT as a consolidation of first-line therapy in MCL (see Table 2). In the Eastern Co-operative Oncology Group (ECOG) study,16 56 patients were subjected to brief chemotherapy (four cycles of CHOP-R) before ibritumomab. Only toxicity and response assessment data have been presented so far: RIT increased the overall RR from 72 to 84%, and tripled the CR rate from 14 to 45%. In a European PLRG trial, 20 patients were entered either at diagnosis or in PR (but not resistance) after first-line therapy. A fludarabine-based regimen was applied (three to six cycles of FCM-R) with further ibritumomab consolidation if an adequate cytoreduction was achieved. It was defined as a BM infiltration <20%, LN diameter <3cm and splenomegaly <15 cm. Radioimmunotherapy increased the CR rate over four-fold, from 20 to 85%, and most patients remained in remission for the next 1.5 years (see Figure 1). These early, fascinating results need a longer follow-up and must be confirmed in a multicentre phase III study, perhaps in the third generation of EMCL Network trials.
Conclusions
Radiotherapy is an active treatment modality in MCL. Ibritumomab radioimmunotherapy is an interesting alternative to other consolidation methods. In younger patients subjected to intensive chemotherapy followed by autologous transplant, it may be an element of a transplantconditioning regimen (i.e. Z-BEAM). In elderly patients, the role of zevalin consolidation should be further investigated in a phase III trial.