Although Hodgkin’s lymphoma (HL) is considered one of the most curable human cancers, the treatment of patients with relapsed and refractory disease, especially those who relapse after autologous stem cell transplantation (ASCT), remains challenging.1,2
Furthermore, because the median age of patients is in the mid-30s, the impact of early mortality on the number of years lost from productive life is remarkable. At the present time, patients with HL whose disease relapses after stem cell transplantation have no curative options. New drugs and novel treatment strategies that are based on our understanding of the disease biology and signalling pathways are needed to improve treatment outcomes in these patients. Ironically, because HL is a rare disease and has long been considered highly curable, the search for new therapies and treatment approaches has grown slowly in the past, with no new drugs approved in the last 30 years.
With recent advances in our understanding of HL pathology, biology and immunology, several therapeutic targets have been identified and are currently under preclinical and clinical investigation. Some of these new compounds have already been evaluated in large Phase II clinical trials seeking potential approval by regulatory agencies. This article focuses on current treatment strategies for patients with relapsed and refractory HL, including the role of stem cell transplantation and promising new monoclonal antibodies and small molecules.
Role of Stem Cell Transplantation Autologous Stem Cell Transplantation
The superiority of high-dose chemotherapy with stem cell rescue over standard second-line chemotherapy alone was demonstrated in two randomised trials.3,4 About half of all patients undergoing ASCT are rescued and definitely cured by such an approach, but the outcome in patients relapsing or refractory to second-line chemotherapy and ASCT is dismal, with a median survival of less than three years.5 The need to identify patients who will not benefit from ASCT has resulted in a large number of studies describing various prognostic factors, including stage and time to relapse, B symptoms, extranodal disease, bone marrow involvement and chemosensitivity at relapse. Depending on the presence or absence of these factors, progression-free survival (PFS) and overall survival (OS) rates range from 10 to 83 % and from 13 to 90 %, respectively (see Table 1).6–12 So, the ideal patient who will benefit from ASCT is the one with limited stage, long time to relapse and chemosensitive disease, while the patient with advanced stage, short time to relapse and refractory disease has the worst prognosis. A recent report13 on the role of tandem ASCT suggests a potential OS and PFS benefit for poor-risk patients – with reported five-year PFS and OS of 36 and 45 %, respectively, in patients resistant to second-line chemotherapy – and these results compare favourably with other published series of patients refractory to second-line chemotherapy.12One of the most important and widely accepted prognostic factors for patients undergoing ASCT appears to be chemosensitivity at relapse,with patients responding to second-line chemotherapy having a much better outcome than patients with refractory disease, whose relapse rate approaches 80 % in some published series.9–12 The concept of chemosensitivity is strictly related to the imaging techniques and criteria used to assess the response to therapy. The advent of functional imaging techniques such as fluorodeoxyglucose-positron emission tomography (FDG-PET) has further improved the reliability of the response evaluation, allowing the early detection of chemoresistance. In fact, the FDG-PET scan after two cycles of chemotherapy (PET-2) is now considered the most powerful prognostic predictor in the first-line setting.14 Retrospective studies have suggested that this concept also applies to relapsed or refractory patients undergoing second-line chemotherapy and ASCT, but this finding needs to be confirmed in prospective studies (see Table 2).14,16–22
Allogeneic Stem Cell Transplantation In clinical practice, allogeneic stem cell transplantation (allo-SCT) has so far been offered to patients relapsing after ASCT. The evidence for a graft-versus-lymphoma (GVL) effect is indirect and comes from:
- retrospective comparisons between relapse rates of allo-SCT recipients and ASCT recipients;
- the fact that several groups reported disease regressions following donor lymphocyte infusions; and
- reports on the protective effect of chronic graft-versus-host disease (GVHD).23
The treatment-related mortality observed with myeloablative conditioning regimens remains high and outweighs any survival advantage compared with ASCT.24 Reduced-intensity conditioning (RIC) regimens are currently being explored with promising results (see Table 3).24–30
In a recent paper from the European Group for Blood and Marrow Transplantation, the outcome in 89 HL patients who received RIC was compared with that in 79 patients who received myeloablative conditioning. The relapse rate was higher in the group receiving RIC (57 compared with 30 %), but the OS rate significantly favoured the RIC group (28 compared with 22 %), due to a much lower non-relapse mortality.24
Although the type of donor – matched related (MRD) or unrelated donor (URD) – does not seem to affect the prognosis, in a recent study, a consistent relapse-free survival advantage has been reported for haploidentical stem cell transplantation over MRD and URD.31
Monoclonal Antibodies XmAb2513 and SGN35 – Targeting CD30
CD30, a member of the tumour necrosis factor (TNF)-receptor superfamily, is an attractive target, because it is expressed nearly universally in Hodgkin’s and Reed–Sternberg (HRS) cells, with an extremely limited pattern of expression in normal tissues.32,33
Results from two clinical studies using first-generation naked anti-CD30 monoclonal antibodies in patients with relapsed HL have been disappointing, perhaps reflecting their poor antigen-binding and/or effector cell-activation properties.34 A second-generation humanised anti-CD30 antibody, XmAb2513, was recently developed to improve antigen binding and Fc-gamma receptor IIIA affinity and specificity.35,36 A Phase I study of XmAb2513 is currently enrolling patients in the US and encouraging preliminary results, with some responses, were recently reported.37
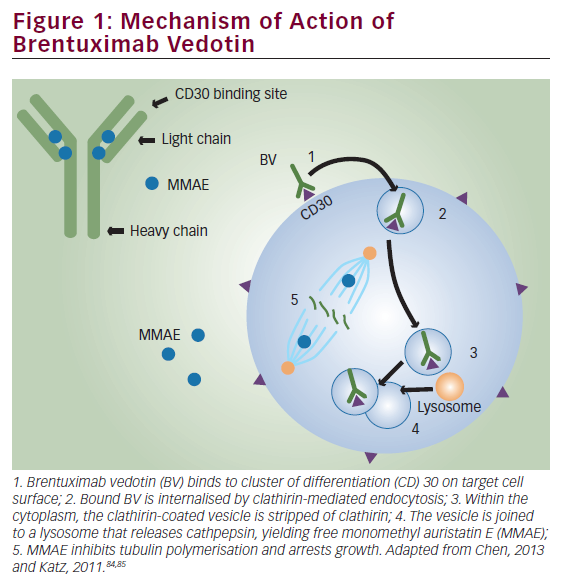
In an alternate strategy, the anti-CD30 antibody cAC10 was conjugated to a synthetic anti-microtubule agent, monomethylauristatin E (MMAE), resulting in a novel immunotoxin conjugate called SGN35.38 SGN35 was recently evaluated in two Phase I clinical trials in patients with relapsed HL and anaplastic large-cell lymphoma (ALCL). In the first study, SGN35 was administered by intravenous infusion every three weeks. Of 42 patients with relapsed/refractory disease, 17 (40 %) achieved partial or complete remission and 86 % of the patients had documented tumour reductions.39 A second Phase I study evaluated the safety and efficacy of SGN35 given on a weekly schedule. Of 17 evaluable patients, seven achieved complete response (CR) and one achieved partial response (PR), an overall response rate of 47 %.40 On the basis of this encouraging clinical activity, SGN35 is currently being evaluated in two Phase II pivotal trials seeking Food and Drug Administration (FDA) approval in patients with relapsed HL and ALCL.
Rituximab – Targeting CD20
The anti-CD20 monoclonal antibody rituximab was evaluated in the treatment of patients with classical HL. Although CD20 antigen is infrequently expressed by HRS cells, it is highly expressed by the reactive B cells in the microenvironment. Thus it was hypothesised that rituximab may induce clinical remission in classical HL by depleting B cells from the microenvironment, by directly killing the few cases of CD20-expressing HRS cells and perhaps by killing the putative HRS CD20-positive stem cells.41 In a pilot study, investigators from the University of Texas MD Anderson Cancer Center treated 22 patients with relapsed classical HL with six weekly doses of rituximab; of these 22 patients, six demonstrated CD20 expression by HRS cells.42 Five patients (23 %) achieved partial or complete remission and eight additional patients had stable disease (SD). Clinical remission was observed in patients regardless of CD20 expression by HRS cells and was limited in patients whose disease was confined to the lymph nodes.In a subsequent study, the same investigators combined rituximab with adriamycin–bleomycin–vinblastine–dacarbazine (ABVD) chemotherapy to treat patients with newly diagnosed classical HL.43 Fifty-two patients with newly diagnosed classical HL were treated in a Phase II study. With a median follow-up of 32 months, the estimated event-free survival (EFS) was 82 % and OS was 100 %. Importantly, the EFS improved for all risk categories: for patients with an internationally prognostic score of 0 to 1, the EFS was 92 %; for scores 0 to 2, 86 %; and for scores 3 to 5, 73 %. These findings are currently being confirmed in a multicentre, randomised study comparing ABVD with rituximab plus ABVD.
Daclizumab – Targeting CD25
CD25 is the interleukin-2 receptor (IL-2R) alpha subunit. IL-2R is a growth factor receptor linked to a variety of signalling pathways – Janus kinase/signal transducer and activator of transcription (Jak-STAT), AKT/mammalian target of rapamycin (mTOR) and mitogen-activated protein (MAP) kinase signalling – involved in inflammation, cell growth and proliferation. It is a transmembrane receptor protein present on only a few normal cells, such as activated T cells, activated B cells, some thymocytes and myeloid precursors, but also expressed in T-cell and B-cell malignancies, such as adult T-cell leukaemia, cutaneous T-cell lymphoma, ALCL and hairy cell leukaemia and on Reed–Sternberg and associated polyclonal T cells in HL. Abnormalities in CD25 expression have also been found in autoimmune diseases, allograft rejection and GVHD.45
The humanised anti-CD25 monoclonal antibody daclizumab was first approved by the FDA in 1997 for use in the prevention of renal allograft rejection. Putative mechanisms of action of daclizumab are blockade of IL-2R alpha signalling, a mechanism involving the FcR receptor and antibody-dependent, cell-mediated cytotoxicity (ADCC), and the recently documented rise of natural killer cells in the peripheral blood in humans.46–49 Daclizumab was proven to be effective and was mainly investigated in T-cell malignancies such as adult T-cell leukaemia/lymphoma and ALCL.45 Given the expression of CD25 in Reed– Sternberg cells, daclizumab was also tested in HL. A Phase I/II trial used the immunoconjugate composed of daclizumab and the Pseudomonas aeruginosa toxin PE38 in 59 patients with leukaemia/lymphoma expressing IL-2R alpha. Eight patients showed objective responses, one of whom was affected by HL.50 In a recent Phase II trial, daclizumab conjugated with the radionuclide yttrium-90 (90Y-daclizumab) was investigated in 30 relapsed/refractory HL patients.51 Radioimmunotherapy with 90Y-daclizumab was given once every six weeks at a dose of 15 mCi for a maximum of seven cycles. Twelve patients achieved CR, seven achieved PR and five had SD. The main side effects were haematological, with prolonged thrombocytopenia, as well as three patients who developed a myelodysplastic syndrome following treatment. These encouraging results, if confirmed in larger numbers of patients, make 90Y-daclizumab one of the most active new investigational drugs in relapsed/refractory HL.
Novel Small Molecules
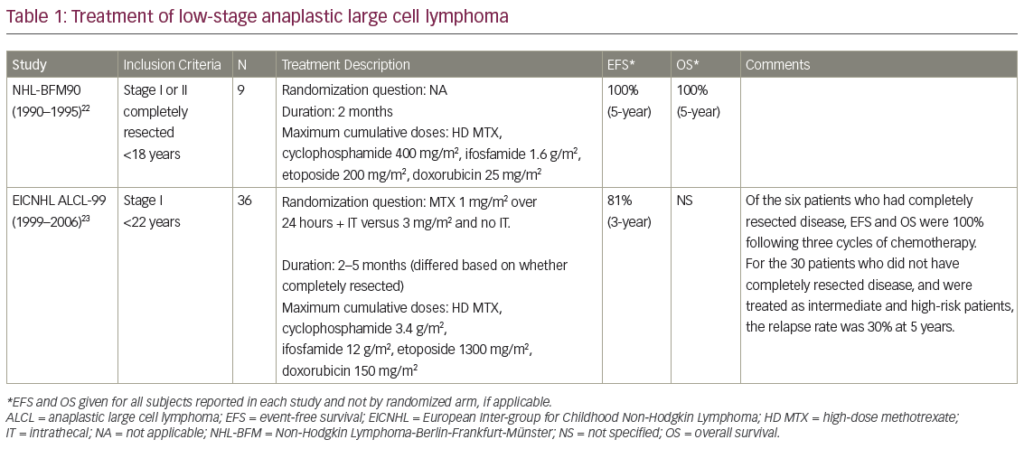
HRS cells aberrantly express a variety of pro-survival proteins, such as nuclear factor-kappaB (NF-kappaB), Jak/STATs, Akt/mTOR, Notch-1 and extracellular signal-regulated kinase (ERK), that can be targeted by small molecules.52–54 These proteins can be targeted either by selective small-molecule inhibitors – Jak-2, mTOR and B-cell lymphoma (Bcl)-2 family inhibitors – or by broad inhibitors that modulate several unrelated molecules, such as histone deacetylase (HDAC), proteasome and heat shock protein 90 (HSP90) inhibitors (see Figure 1).
Histone Deacetylase Inhibitors
It is well established that post-transcriptional histone modification plays an important role in regulating gene transcription and that such modification is mediated by several enzymes, including histone acetyltransferases (HATs) and HDACs.55 The balance between HATs and HDACs is critical for regulating the expression and functional status of a variety of proteins involved in cell proliferation, survival, angiogenesis and immunity.56–60
Eighteen HDACs have been identified in humans and grouped into two major categories: zinc-dependent HDACs and nicotinamide adenine dinucleotide (NAD)-dependent HDACs.55,56,71 Furthermore, HDACs are classified into four major classes: class I (HDAC 1, 2, 3), class II (HDAC 4, 5, 6, 7, 9 and 10), class III (sirtuin 1–7) and class IV (HDAC 11). Class III is NAD-dependent, whereas classes I, II and IV are zinc-dependent.
MGCD0103 and etinostat (SNDX-275, formerly MS-275) preferentially inhibit class I HDACs (isotype-selective HDAC inhibitors). Vorinostat, ITF2357 and panobinostat (LBH589) inhibit HDAC classes I and II (pan-HDAC inhibitors) (see Table 4).
MGCD0103 is a novel oral non-hydroxamate benzamide-based HDAC inhibitor that selectively inhibits HDAC 1 and 2 (and, to a lesser extent, 3 and 11) isoforms. The safety and efficacy of MGCD0103 given orally three times per week (85–110mg starting doses) were recently evaluated in a study in patients with relapsed and refractory HL.61 Of 20 patients treated at the 110 mg dose level, seven (35 %) achieved partial or complete remission. However, this dose level was poorly tolerated, resulting in dose interruptions and reductions and discontinuation of therapy. Subsequently, three (30 %) of 10 patients enrolled on a reduced dose (85 mg) achieved partial remission, and the treatment was well tolerated. Overall, 80 % of the 30 evaluable patients had some decrease in their tumour measurements. Three pan-HDAC inhibitors were recently evaluated in patients with relapsed HL. In the first study, panobinostat was evaluated in a Phase I trial in patients with haematological malignancies that included relapsed HL.62 Five of 13 (38 %) HL patients achieved partial remission. On the basis of this promising clinical activity, two large international Phase II studies of panobinostat in relapsed HL are now enrolling patients to confirm these results.63,64 In a second study, the Southwest Oncology Group (SWOG) conducted a Phase II trial of vorinostat in patients with relapsed HL.65 Twenty-five patients were treated with 200 mg vorinostat given orally twice a day for 14 days every 21-day cycle. Unlike MGCD0103 and panobinostat, vorinostat produced only modest clinical activity, as only one patient (4 %) achieved partial remission.
Vorinostat was shown to induce cell cycle arrest and apoptosis in HL cell lines and to synergise with chemotherapy.66 Furthermore, vorinostat inhibited STAT6 phosphorylation and transcription in HL cell lines, an effect that was associated with a decrease in the expression and secretion of T-helper 2 (Th2)-type cytokines and chemokines, including thymus and activation-regulated chemokine (TARC/CCL17) as well as interleukin-5 and an increase in T-helper 1 (Th1)-type cytokines and chemokines, including a profound increase in IP-10 levels.66 This study confirms that HDAC inhibitors can also determine changes in the tumour microenvironment.
ITF2357 is a selective class I and class II HDAC inhibitor. The efficacy, safety and tolerability of daily 100 mg doses of ITF2357 in relapsed/refractory HL were investigated in a Phase II clinical trial at the National Tumour Institute of Milan. The preliminary results were presented at the 2008 American Society of Clinical Oncology (ASCO) meeting. Fifteen patients were enrolled and 13 were evaluable for response. Seven patients (54 %) had a stable disease, with a reduction in FDG-PET uptake in six patients (46 %) lasting at least three months; six patients had disease progression. Interestingly, a correlation was found between a decrease in serum TARC levels and the response to treatment in this study.67 On the basis of the single-agent activity of ITF2357 and the documented synergistic activity of ITF2357 and mechlorethamine in HL cell lines, a Phase II trial of ITF2357 combined with mechlorethamine was conducted by the same group. Nineteen patients who unsuccessfully underwent prior ASCT/allo-SCT were enrolled and preliminary data were presented at the 2008 American Society of Hematology (ASH) meeting. Seventeen patients were evaluable for response, with two CR (12 %), three PR (18 %) and five SD (29 %). The main toxicity was haematological, with seven patients experiencing grade 3/4 neutropenia and eight having thrombocytopenia; four patients experienced infections during treatment.68 Taken together, these data suggest that ITF2357 has encouraging clinical activity in relapsed/refractory HL.
Mammalian Target of Rapamycin Inhibitors
The phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signalling pathway (see Figure 1) is one of the most aberrantly activated survival pathways in cancer, making it an important target for drug development.69,70 This pathway is negatively regulated by the tumour-suppressor protein phosphatase and tensin homologue (PTEN). In vitro experiments demonstrated that inhibition of PI3K, Akt or mTOR by various small molecules can induce cell cycle arrest, autophagy and apoptosis in HRS-derived cell lines.71–73 In addition to a direct antitumour effect, mTOR inhibitors may induce clinical responses by enhancing the immune response and inhibiting angiogenesis.74,75
The therapeutic value of inhibiting the PI3K/Akt/mTOR axis has recently been studied using the oral mTOR inhibitor everolimus in 17 relapsed-refractory patients (intention to treat analysis) (see Figure 2).76 Of 15 evaluable patients with relapsed HL treated daily with 10 mg everolimus, seven (47 %) achieved partial responses (see Figure 2 and Table 5). If this finding is confirmed in a larger number of patients, everolimus may become one of the most active agents in relapsed HL. In vitro experiments suggested that mTOR inhibitors may synergise with chemotherapy, PI3K inhibitors and HDAC inhibitors in a variety of tumour models, including HL.71,77 A Phase I clinical trial combining the HDAC inhibitor panobinostat with the mTOR inhibitor everolimus is currently enrolling patients with non-Hodgkin’s lymphoma (NHL) and HL.
Proteasome Inhibitors
NF-kappaB plays a central role in regulating the expression of various genes involved in cell survival, apoptosis, carcinogenesis and inflammation, making it a potential therapeutic target. The first attempt to therapeutically inhibit NF-kappaB activation in HL used the proteasome inhibitor bortezomib. By inhibiting the degradation of cytoplasmic I-kappaB-alpha, bortezomib inhibits the activation of NF-kappaB.78 In preclinical studies, bortezomib inhibited HL cell line proliferation and induced cell cycle arrest and apoptosis in a time-dependent and dose-dependent manner and was effective even in HL cell lines that harboured mutations in the I-kappaB-alpha gene.79 Furthermore, bortezomib enhanced the effect of gemcitabine chemotherapy and potentiated the effect of anti-CD30 antibody and tnf-related, apoptosis-inducing ligand (TRAIL)/Apo2L.79,80 Despite these favourable preclinical results, bortezomib demonstrated no significant clinical activity in patients with relapsed HL.81.82 On the basis of these preclinical experiments, bortezomib-based combinations were evaluated in patients with relapsed classical HL. In the first study, a Phase I trial evaluated the combination of bortezomib with the ifosfamide–carboplatin–etoposide (ICE) regimen in relapsed/refractory HL.83 Bortezomib was given at doses of 1, 1.3 or 1.5 mg/m2 on days one and four of each ICE cycle. Twelve patients were enrolled, of whom six achieved PR and three achieved CR, for an overall response rate of 75 %. On the basis of these encouraging data, a randomised Phase II study comparing ICE with bortezomib plus ICE is currently enrolling patients to determine the contribution of bortezomib to the ICE regimen.
In a second study, 18 patients with relapsed HL were treated with bortezomib in combination with gemcitabine.84 Because of the relatively low response rate (22 %), coupled with treatment-related liver toxicity, the authors concluded that this regimen should not be further developed for the treatment of HL.
Heat Shock Protein 90 Inhibitor 17-AAG
Heat shock proteins are cellular chaperone proteins that are required for essential housekeeping functions such as protein folding, assembly and transportation across different cell compartments. HSP90 is frequently overexpressed in cancer cells and hence is an attractive target for cancer therapy. Similarly to findings in other cancers, HSP90 is overexpressed in primary and cultured HL cells.85,86 HSP90 chaperones several client proteins that promote HRS cell survival, including ERK, Akt and NF-kappaB.52,80,87
In a preclinical experiment, the HSP90 small-molecule inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) down-regulated Akt and cellular FLICE-inhibitory protein (cFLIP) and induced apoptosis in HL-derived cell lines.87 Furthermore, 17-AAG synergised with doxorubicin and agonistic anti-TRAIL death receptor antibodies. On the basis of these findings, a Phase II study of 17-AAG is currently enrolling patients with relapsed HL, and the results are expected to be available in the near future.
Lenalidomide
Two independent groups have evaluated the safety and efficacy of lenalidomide in patients with relapsed HL. In the first study, Fehniger et al. reported their experiences with 25 mg/day lenalidomide on days 1–21 of a 28-day cycle.88 Four of the 12 evaluable patients responded (one CR and three PR). Grade 3 and 4 neutropenia was observed in 47 % and thrombocytopenia was seen in 27 % of the patients. In a second study, Kuruvilla et al. treated 15 patients with relapsed HL using the same dose and schedule of lenalidomide as in the previous study.89 Two patients achieved PR and seven achieved SD, with a median time to progression of 3.2 months. Collectively, these data suggest that lenalidomide has promising single-agent activity in relapsed HL.
Conclusion
After more than three decades of quiet on the drug development front, several compounds have been identified as promising agents for the treatment of patients with relapsed classical HL. Future research should focus on identifying biomarkers, selecting patients who are likely to respond to these novel agents, incorporating these new agents with existing effective regimens and identifying predictive markers for treatment response. Ultimately, randomised clinical trials will be required to document the impact of these new agents on patients’ survival.