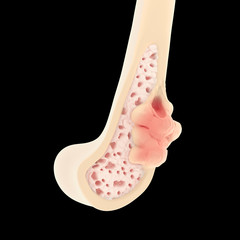
Giant cell tumor of bone (GCT) is an uncommon, benign condition that typically affects patients of both sexes between 20 and 40 years of age. GCT accounts for 15–20% of all benign bone tumors.1 GCT is more common in ethnic Chinese populations, and cases of multifocal and familial GCT suggest that inherited factors may play a role.2 The genetic basis of GCT is not known, and no risk factors have been described. The tumor is characterized by localized bone destruction, leading to swelling, pain, and fracture. The most commonly affected sites include the distal femur, proximal tibia, and distal radius, but almost any bone may be involved. GCT typically presents as an intraosseous lytic lesion, but may extend into adjacent soft tissues. In epiphyseal regions, the bony destruction may extend to the cartilage margin, resulting in joint instability due to lack of subchondral bone. In the pelvis and spine, soft-tissue extension may cause neurological complications. Despite the notionally benign nature of GCT, pulmonary metastases may occur in some patients, which usually do not progress. Frank malignant transformation occurs in 2–3% of GCT, and is associated with clinical behavior typical of high-grade sarcomas.1
Pathologically, GCT belongs to a spectrum of benign, giant-cell-rich tumors, which includes giant cell tumor of tendon sheath, pigmented villonodular synovitis, aneurysmal bone cyst, and giant cell reparative granuloma.1 GCT is characterized by a stromal cell population, which is thought to be a mesenchymal osteoblast precursor, and variable numbers of eponymous giant cells. The giant cells are CD4+ and CD68+, positive for tartrate-resistant acid phosphatase and other markers of true osteoclasts, which is consistent with their hemopoietic origin. Mononuclear osteoclast-like cells can also be observed, which presumably represent precursor cells. The mesenchymal cell population is typically mononuclear and expresses vimentin. It is thought that the mesenchymal cell is the neoplastic component of GCT.1 Treatment of GCT depends on the stage of the disease, and is generally based on uncontrolled and retrospective studies (reviewed by Thomas and Skubitz2). In most cases, definitive treatment involves surgical curettage.3 Local adjuvant therapies may be employed to reduce the risk for relapse. Such adjuvant treatments include packing the cavity with bone cement, where the thermogenic polymerisation of methylmethacrylate may kill residual tumor cells.4 The local relapse rates for curettage vary between 20 and 50%. En bloc excision of tumors may also be used, with appropriate reconstruction of the adjacent joint.3 This process, which carries a higher morbidity, reduces the relapse rates to about 10%. Surgical excision may be associated with unacceptable morbidity, for example with large sacral lesions, lesions affecting the spine, large lesions extending into soft tissues, or metastatic lung lesions. Radiotherapy may be useful, and is associated with 60–80% local control rates.5 However, in addition to other long-term morbidities associated with radiotherapy in young patients, concerns have been raised in terms of an increased risk for malignant transformation.6 The risk for malignant transformation may be lower with more recent megavoltage techniques. Arterial embolization has also been used for local control.7 To date, there are no effective systemic therapies for unresectable GCT. Agents that have been reported, usually in retrospective case series, include interferons, ifosfamide, doxorubicin, cyclophosphamide, and cisplatin (reviewed by Thomas and Skubitz2). Given the associated toxicity, the lack of obvious clinical activity, and the benign nature of GCT, none of these treatments can be regarded as standard of care. Bisphosphonates have been used with some effect in retrospective case series, with reduction in relapse rates reported.8,9 Interestingly, giant cells were not eradicated by the use of bisphosphonates.9
Bone Biology and RANKL
Bone is a complex, dynamic structure undergoing constant remodeling throughout life (reviewed by Martin et al.10 and Sims et al.11). Excess bone resorption is a major contributor to bone and joint diseases.12 Osteoporosis, or generalized loss of bone mass, is a common disease and a significant health burden. The major problems that arise are due to fractures in the elderly, leading to immobility and a substantial mortality. Osteoporosis is due to an excess of bone resorption over formation, and is most commonly seen in women. In general, peak bone mass is established relatively early in adult life, and thereafter declines gradually with age. This decline is accelerated by the loss of estrogen following menopause in females. A second common clinical scenario associated with bone loss is in the setting of malignant disease. Typically, bone metastases from breast, prostate, lung, and kidney cancers will result in localized bone destruction (and to some extent bone formation), which causes pain and an increased risk for fracture, particularly in weight-bearing regions. In some malignant diseases, such as multiple myeloma, the bone loss may be generalized. While the osteoblast lineage has been reasonably well characterized, the osteoclast lineage was for many years less well understood (recently reviewed by Bar-Shavit13 and Boyce et al.14). It was clear that the osteoclast was responsible for bone resorption. Morphologically, the osteoclast was a mono- or multinucleated cell, found almost exclusively at bone surfaces. Multinucleated osteoclasts may contain up to 50 nuclei. Markers of the osteoclast lineage include expression of tartrate-resistant acid phosphatase, cathepsin K, and abundant adenosine triphosphate (ATP)-dependent proton pumps located in the plasma membrane of osteoclasts adjacent to the bone surface. Bone resorption is effected by a combination of acidification of the compartment under the osteoclast, and by the secretion of a cocktail of proteases. Early animal studies demonstrated that osteoclasts (but not osteoblasts) could be regenerated following lethal irradiation by circulating cells from a donor, suggesting a hemopoietic origin.15–17 Subsequent studies established that osteoclasts could be generated in vitro by co-culture of bone marrow stromal cells with cells of the monocyte lineage, confirming this hypothesis and demonstrating an inter-dependence between the osteoblast and osteoclast lineages.18 These studies showed that signaling molecules, including parathyroid hormone (PTH), PTH-related protein, vitamin D, and prostaglandins, acted via the osteoblast to increase osteoclast formation in vitro.19 However, all of these agents required co-culture with osteoblasts, suggesting the presence of an effector molecule that may represent a final common pathway downstream of these various signals. In 1997, it was demonstrated that this common signal was receptor activator of NF κβ ligand (RANKL), identified as a high-affinity ligand for the tumor necrosis factor (TNF) receptor, RANK.20–22 Both RANK and RANKL, also known as TNF-related activation-induced cytokine (TRANCE), osteoprotegerin (OPG) ligand (OPGL), and osteoclast differentiation factor (ODF), are essential for osteoclast development in vivo by targeted disruption.23,24 In addition, a soluble, non-membrane- associated form of RANK exists: OPG, which is thought to act as a decoy receptor for RANKL, thereby acting as an endogenous inhibitor of RANKL signaling.24,25 Monocytes could be differentiated into osteoclast-like cells in vitro without osteoblasts, implying that RANKL was the final common osteoblast-derived signal mediating the osteoclastogenic effects of multiple other signaling pathways.26,27 RANKL expression was observed in vivo in stromal cells within bone.28 It is important to note that other biological functions for RANKL have been identified, including mammary gland development, T-cell activation, and dendritic cell maturation.20,21,29,30
RANKL and Giant Cell Tumor of Bone
Expression of RANKL in GCT of bone was first observed by two groups.31,32 These investigators showed that stromal cells within GCT express RANKL, and that co-culture of stromal cells with the RAW264.7 monocyte-derived cell line resulted in formation of osteoclast-like cells in vitro. OPG was detected in both osteoclast-like cells and the stromal cells within GCT, and RANK was observed primarily in the osteoclast-like cells. Two groups undertook molecular profiling of GCT, and showed that extremely high levels of RANKL and markers of the osteoclast lineage were expressed in GCT.33,34 Several groups identified expression of genes consistent with a pre-osteoblastic origin of the stromal cells in GCT.31,35,36 These data together suggested that an immature osteoblast-derived neoplastic cell initiates GCT of bone by high levels of expression of RANKL. To date, there are no genetic events that are linked to RANKL expression in the stromal compartment of GCT. Interestingly, RANKL is expressed in several osteoclast-enriched neoplastic diseases, including GCT of tendon sheath,35 reparative giant cell granuloma,37 and giant-cell-rich hepatoma,38 suggesting that RANKL may be the final common pathway mediating osteoclast-like cell recruitment in a range of giant-cell-rich tumor types.
Denosumab
Atkins and colleagues observed that OPG treatment dose dependently inhibited resorption of bone slices by GCT in culture, and could also inhibit the formation of multinucleated osteoclasts from precursors within the GCT.31,39 These effects of OPG were competitively reversed by increasing concentrations of exogenous RANKL. This observation, and those outlined above, raised the possibility that blockade of RANKL may provide clinical benefit in GCT. However, the use of recombinant OPG itself may be associated with the generation of autoimmunity to the endogenous gene product, as has been observed for patients treated with recombinant human erythropoietin.40 Denosumab is a fully human monoclonal antibody with high affinity for human RANKL of immunoglobulin (Ig)-G2 sub-class (reviewed by Miller41). It has little cross-reactivity with other TNF family members. Studies on over 5,000 patients with post-menopausal or androgen-deprivation osteoporosis have been published. An initial study using denosumab in 412 female patients with post-menopausal osteoporosis (AMG162 study) indicated a profound and sustained effect on suppression of bone turnover, with marked lowering of alkaline phosphatase, and urinary collagen C-telopeptide.42 The investigators observed that serum calcium levels were transiently suppressed to some degree, and this was accompanied by increases in serum PTH levels. There was a strong effect to increase bone mass measured at various sites and total body bone mass in this study. These effects appeared to be to some degree dose-dependent, with a maximum effect seen with 60mg subcutaneous doses. The authors also noted that a sustained effect on total bone mass was seen with six-monthly dosing, although C-telopeptide levels started to increase by three months, and a greater effect on bone mass was observed with monthly treatments. Two subsequent large-scale randomized trials have been conducted in post-menopausal osteoporosis (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months [FREEDOM])43 and men with non-metastatic prostate cancer receiving androgen-deprivation therapy (Hormone Ablation Bone Loss [HALT] trial).44 Based on the earlier study, a 60mg dose of denosumab was used, administered every six months in the osteoporosis study and monthly in the prostate cancer study. Denosumab given subcutaneously twice yearly for 36 months was associated with a reduction in the risk for vertebral, nonvertebral, and hip fractures in women with osteoporosis, and a reduction in the incidence of new vertebral fractures among men receiving androgen deprivation therapy. Denosumab was generally well-tolerated, with no difference in cancer, infective, or cardiovascular serious adverse events in the FREEDOM or HALT trials. These are important observations given the benign nature of these diseases. Interestingly, the incidence of eczema increased in the denosumab arm of the FREEDOM trial (3% compared with 1.7%), and the incidence of cellulitis also increased (0.3% compared with 0.1%). Also of interest, because of the known association of bisphophonates with osteonecrosis of the jaw (ONJ), no ONJ was observed in the HALT or FREEDOM studies. No neutralizing antibodies were observed.
Denosumab and Giant Cell Tumor of Bone
A proof-of-principle study of denosumab in GCT has recently been reported.45 The target population were patients with recurrent or unresectable GCT. Treatment consisted of 120mg of denosumab, administered subcutaneously for three consecutive weeks in the first month, and monthly thereafter. The primary end-points of the study were safety and tolerability in this population of patients, and efficacy as assessed by >90% eradication of giant cells on treatment or evidence of stable disease at six months. Secondary end-points included monitoring the development of neutralising antibodies and markers of bone turnover. No formal subjective or quality of life data were incorporated into the study end-points. A total of 37 patients were enrolled.45 Of 35 evaluable patients, 30 (86%) met the criteria for response. Of all patients for whom histological data are available, all met the histological criteria for response, while 10 of 15 patients met radiological criteria for response. Interestingly, bone repair was observed in nine patients. Although not a part of formal study requirements, investigators reported assessment of clinical benefit in 31 patients, of whom 26 experienced reduced pain or improvements in functional status. As previously noted, suppression of bone turnover was observed. In general, the treatment was well-tolerated. One death on study was observed, which was not treatment-related. These promising data suggest that denosumab offers a new option for treatment of patients with previously untreatable GCT, and offers insights into the application of denosumab in younger patients than have been previously studied. Of course, many important questions remain to be answered. We do not know how long treatment needs to continue, or whether it will be possible to stop treatment in some patients, and if so, in whom. We do not know whether denosumab will have a role in patients with resectable disease, perhaps as an adjuvant or neoadjuvant treatment that may reduce the risk for relapse, or reduce the scale and morbidity of surgery. Another important question is whether denosumab will have a role in other giant-cell-rich tumors where RANKL appears to play a role. Finally, we do not yet know the long-term effects of treatment with denosumab, particularly in younger patients, and the follow-up of these patients will be of interest.
Summary
There are no effective systemic therapies for unresectable or recurrent GCT. Thirty years of basic research has defined the cellular and molecular biology of bone, and particular the close inter-relationship between the osteoblast and the osteoclast and the critical role in bone of RANKL. Recent evidence suggests that RANKL plays a major role in GCT, as it is expressed by the neoplastic stromal component and mediates recruitment of the monocytic precursors that then form osteoclast-like cells and erode bone. The development of denosumab, a fully human monoclonal antibody to RANKL, has led to a clinical trial in unresectable and recurrent GCT. The interim results suggest that denosumab may provide clinical benefit for patients with few other options. The final role of denosumab in giant-cell-rich disorders is currently under investigation. ■