Ovarian cancer is the fifth most common malignancy in women. In the US, approximately 22,000 new cases occur each year.1 In Germany, about 8,000 new cases are diagnosed each year. About 80% of patients suffer from advanced-stage disease (International Federation of Gynaecology and Obstetrics [FIGO] III/IV) with an unfavorable prognosis. Due to the lack of early specific symptoms, most patients are diagnosed at an advanced stage when the cancer has spread beyond the pelvis. Consequently, only 20% of patients are diagnosed at a stage where the malignancy is still confined to the ovary or lower pelvis. These cases, which carry a good prognosis with a five-year survival of about 90%, are often accidentally diagnosed when the ovaries are removed due to cystic changes.
At present, there is no valid screening tool available for ovarian cancer. Sequential vaginal ultrasound is not reliable, even in high-risk patients, because of breast cancer (BRCA) I or II mutations. Also, no blood test, whether the cancer antigen (CA)-1252 or any other protein profiling,3 displayed sufficient specificity or sensitivity to be used as a routine screening marker for ovarian cancer. We have recently shown that the combination of several secreted markers (osteopontin, kallikrein, or matrix metalloproteinase [MMP]7) in combination with CA-125 can accurately distinguish sera of ovarian cancer patients from those of healthy women. Although this blood test was also able to detect early-stage disease, further validation is still necessary.4 The most important prognostic marker for survival in advanced ovarian cancer patients remains the post-operative tumor burden.5 No medical therapy is able to compensate for an inadequate surgical procedure; therefore, patients suspected of ovarian cancer should be referred to a specialized hospital site.
In 1996, the Gynecology Oncology Group (GOG) study 1116 showed that cisplatin in combination with paclitaxel was superior to cisplatin and cyclophosphomide with respect to disease-free survival (DFS) and overall survival (OS). This study was conducted in patients who had received a suboptimal tumor reduction surgery. DFS and OS were significantly prolonged in those patients who received cisplatin and paclitaxel comparedwith patients who received cisplatin and cyclophosphomide (18 versus 12.9 months and 37 versus 24 months, respectively). Based on these results, three prospective randomized trials proved the efficacy of paclitaxel in combination with platin derivates.7–9 The combination of cisplatin, paclitaxel, carboplatin, and paclitaxel showed similar values for time to progression and OS. In contrast, the latter combination was less neurotoxic than the cisplatin-based regimen. Therefore, quality of life is significantly improved by using carboplatin instead of cisplatin.10 Another advantage in favor of the carboplatin/paclitaxel combination is that this regimen can be administeredin an outpatient setting. Therefore, today’s standard primary chemotherapy in ovarian cancer treatment is the combination of carboplatin and paclitaxel, providing the best ratio of benefits to side effects.11
Although this gold standard has been improving OS significantly, the prognosis of ovarian cancer remains poor due the occurrence of chemotherapy resistance. One option to improve the efficacy of the standard treatment is the incorporation of non-cross-resistant cytotoxic drugs in addition to carboplatin and paclitaxel. Several attempts have been undertaken over the past few years with different drugs. The addition of epirubicine did not prolong progression-free survival (PFS) or OS, but did increase hematological and non-hematological toxicities.12,13 Also, the combination of carboplatin, paclitaxel, and topotecan as sequential single agents did not improve outcome.14,15 Incorporating liposomal pegylated doxorubicine and gemcitabine did not benefit patient survival.16 So far, all attempts to further improve efficacy by adding a third non-cross-resistant conventional cytotoxic agent (e.g. topotecan, etoposide, pegylated liposomal doxorubicine, or gemcitabine) have failed.
Using an alternative method to administer the chemotherapeutic agents may optimize treatment. In one recently published study (GOG172),17 patients were treated with chemotherapy administered intravenously (IV) and intraperitonealy (IP). Patients treated in the standard arm received the combination cisplatin 75mg/m2 and paclitaxel 135mg/m2 IV. In contrast, patients treated in the experimental arm received paclitaxel 135mg/m2 on day one IV, cisplatin 100mg/m2 IP on day two, and paclitaxel 60mg/m2 IP on day eight. The authors report a significantly higher median survival for the ‘IP group’: 65.6 versus 49.7 months in the ‘IV group.’ No significant difference was found in PFS, but the patients treated in the IP arm did have a significant disadvantage with respect to quality of life (e.g. abdominal pain, neurotoxicity). Even in experienced cancer centers, only 42% received the IP regimen as planned. Eight percent of the patients did not receive IP chemotherapy at all, and in 34% of the patients only one to two cycles of IP chemotherapy were given. However, interpretation of the results is difficult. One point of criticism is that different dosages of cisplatin wereused (75 versus 100mg/m2). Also, the additional application of paclitaxel on day eight raises the question of whether it would be useful to employ a dose-dense schema rather than giving chemotherapy IP. Additionally, it was not an intention-to-treat analysis. No data about second-line treatment are published; this fact seems to be very important since it is well known that second-line treatment has an impact on survival. Thus, this regimen has not been adopted as standard of care.
Future Perspectives
The future of cancer therapy may lie in identifying biological dependenciesof cancer cells that can be specifically targeted. Within the last few years, more and more specific antibodies and small molecules have become available for targeted therapy. For ovarian cancer patients, the correct therapeutic intervention could be a combination of carboplatin and paclitaxel plus a small-molecule drug. Targeting tumor-induced neoangiogenesis may be one option.
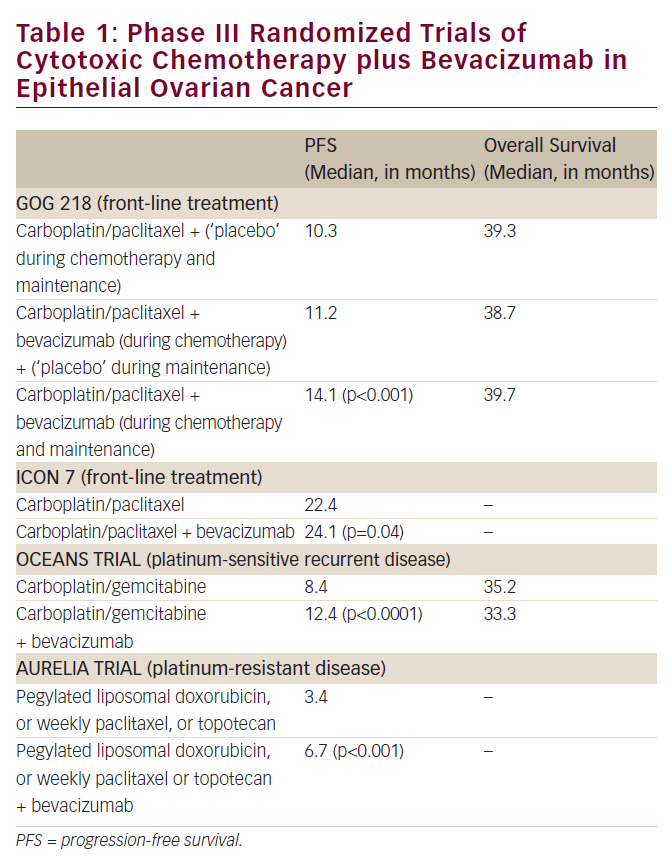
The humanized vascular endolethial growth factor (VEGF) antibody bevacizumab shows clinical responses in heavily pre-treated ovarian cancer patients.18–21 The NCT00129727 trial, in which patients will receive carboplatin, paclitaxel, and bevacizumab, is currently recruiting. In this trial, bevacizumab will be given every three weeks for one year. In a phase II study, erlotinib showed marginal activity but was well tolerated. Interestingly, the survival rate correlated significantly with the skin rush grade induced by erlotinib; patients with higher grades of skin rushes survived for a longer time.22
Erlotinib will also be investigated in a phase III clinical trial, given as maintenance therapy after primary chemotherapy. In phase I clinical trials, the anti-idiotypic antibody abagovomab (CA-125 antigen) in patients withrecurrent ovarian cancer was found to be safe to be administered and was also well tolerated.23,24 Imatinib is a small-molecule drug that inhibits tyrosine kinases. It is used in hematological malignancies and in patients suffering from gastrointestinal stromal tumors (GIST). The recently conducted Southwest Oncology Group (SWOG) S0211 trial using imatinib in recurrent platinum-resistant ovarian cancer did not show a markedly improved response rate.25 Using the small-molecule drug gefitinib in epithelial ovarian cancer also did not show a clinical response. However, at the molecular level a decrease in target phosphorylation was observed.26 Although these data are preliminary, they suggest that the combination of conventional chemotherapy and targeted therapy may increase chemotherapy responses, prolong DFS, and, therefore, improve the prognosis of ovarian cancer.
Conclusion
Today, the combination of radical cytoreductive surgery and chemotherapy with carboplatin and paclitaxel is the standard treatment for advanced ovarian cancer patients. Much hope is pinned on targeted therapies utilizing novel molecular drugs, and several phase III trials with these substances have already begun worldwide.