Trastuzumab deruxtecan (T-DXd) is a highly effective drug for the treatment of human epidermal growth factor receptor 2 (HER2)-positive advanced recurrent breast cancer. It was approved by the US Food and Drug Admninistration even though it had only been through a phase II study. It has attracted a great deal of attention among oncologists, and many clinical trials have been conducted. This article reviews reports of clinical trials and ongoing studies of T-DXd in HER2-positive breast cancer.
Mechanism of action of trastuzumab deruxtecan
T-DXd is a drug moiety, MAAA-1181a, covalently linked to MAAL-9001, a human immunoglobulin G1 (IgGl) monoclonal antibody that is identical in sequence to trastuzumab, via a maleimide tetrapeptide linker. MAAL-9001 is a topoisomerase I inhibitor that is more potent than SN-38 (the active metabolite of irinotecan), which is a derivative of exatecan and can permeate cell membranes. The antibody–drug conjugate (ADC) binds uniformly to MAAA-1181a and exhibits a high drug-to-antibody ratio of 7 to 8.1 T- DXd binds to the HER2 receptor and is then taken up into tumour cells, where it undergoes cleavage by lysosomal enzymes, releasing MAAA-1181a into the cytoplasm.
Preclinical pharmacology studies conducted in vitro found that T-DXd inhibited cell proliferation in a HER2 expression-dependent manner.1 Furthermore, in mouse models of tumour-bearing cancer, T-DXd treatment resulted in the regression of many HER2-expressing tumours, including several models that were refractory to trastuzumab emtansine (T-DM1).2
In additional experiments using mouse models, T-DXd exhibited antitumour activity against HER2-low expressing tumours, suggesting that T-DXd may be effective against HER2-low expressing tumours.1 In a study of mice that were inoculated with a mixture of HER2-expressing and HER2-non-expressing cells, a strong efficacy was observed for T-DXd, but this was not so for T-DM1. The higher efficacy of T-DXd may be due to the bystander effect, affecting adjacent cells, which was caused by the high permeability of the cytotoxic drug that was bound to the cell membrane.1,2
Clinical trial results in HER2-positive unresectable and/or metastatic breast cancer
In a phase I study that was conducted to examine the efficacy and safety of T-DXd in patients with HER2-positive recurrent metastatic breast cancer who were previously treated with T-DM1, T-DXd demonstrated an objective response rate (ORR) of 59.5% and a median duration of response (DOR) of 20.7 months.3 From these results, a study was conducted to evaluate the efficacy and safety of T-DXd. The DESTINY-Breast01 trial produced a recommended dose of 5.4 mg per kg body weight through the pharmacokinetic stage and dose-finding stage, after which patients were enrolled at the recommended dose.4 The trial reported the efficacy of 184 patients who were treated at the recommended dose of 5.4 mg/kg. The backgrounds of these patients included a median of six regimens of prior therapy, all of which included T-DM1 and trastuzumab, 65.8% included pertuzumab and 54.3% included anti-HER2 agents. The median age of the patients was 55.0 years (range 28.0–96.0 years), and the hormone receptor status was positive in 52.7%, negative in 45.1% and unknown in 2.2%.4
Updated results following a median follow-up of 26.5 months (range 0.7–39.1 months) showed a response rate of 62% (95% CI: 534.5–69.0), of which 7.1% presented a complete response (CR) and 54.9% a partial response (PR).5 Progressive disease occurred in only 1.6% of patients.
The median DOR was 18.2 months (95% CI: 15.0–not estimable [NE]), and the median progression-free survival (PFS) was 19.4 months (95% CI: 14.1–25.0). Although the trial targeted heavily pretreated patients, the PFS for T-DXd in this trial was similar to that for trastuzumab-pertuzumab and docetaxel therapy in the Cleopatra trial in first-line patients.6 The median overall survival (OS) was 29.1 months (95% CI: 24.6–36.1) in the DESTINY- Breast01 trial.
DESTINY-Breast02 was a phase III trial comparing T-DXd with physician’s choice of therapy in patients with HER2-positive unresectable metastatic breast cancer who had previously been treated with T-DM1, similarly to the DESTINY-Breast01 trial. The results were presented at the 2022 San Antonio Breast Cancer Symposium.7 In all, 608 patients with HER2-positive advanced breast cancer who had previously been treated with T-DM1 were enrolled, and they were randomized 2:1 to receive T-DXd or physician’s choice of treatment (trastuzumab/capecitabine or lapatinib/capecitabine). The majority of patients had previously received two or three regimens of therapy as treatment following metastatic recurrence. Two prior regimens had been used in 47.3% of the T-DXd group and 45.5% of the group receiving physician’s choice of therapy, and three prior regimens had been used in 30.3% of the T-DXd group and 31.2% of the physician’s choice group. The primary endpoint was PFS evaluation through a blinded, independent, central review (BICR). The secondary endpoints included OS, response rate, DOR and safety. In the analysis, it was found that T-DXd reduced risk of disease progression or death by 64% relative to the investigator’s choice of therapy. The median PFS was 17.8 and 6.9 months for the two groups, respectively. T-DXd also reduced the risk of death by 34%, relative to the physician’s choice of therapy. Median OS terms were 39.2 and 26.5 months, respectively.
DESTINY-Breast03 was an international, multicentre, phase III trial that compared T-DXd with T-DM1 in previously treated HER2-positive patients with recurrent metastatic breast cancer.8 For this trial, a total of 524 patients with HER2-positive unresectable or metastatic breast cancer who had previously been treated with trastuzumab and taxanes were randomized 1:1 to T-DXd or T-DM. The primary endpoint was PFS by BICR, and the main secondary endpoint was OS; other secondary endpoints were ORR by BICR and investigator determination, PFS by investigator determination, and safety.
Updated results were reported at the 2022 San Antonio Breast Cancer Symposium, with data from a median follow-up of 28.4 months for the T-DXd arm and 26.5 months for the T-DM1 arm.9 Median PFS, the primary endpoint, was 28.8 months for T-DXd and 6.8 months for T-DM1. Analysis of the main secondary endpoint showed a statistically significant improvement in OS with trastuzumab T-DXd relative to T-DM1, with a 36% reduction in risk of death. The ORR was 78.5% for T-DXd and 35.0% for T-DM1, and the median DOR was 36.6 months for T-DXd and 23.8 months for T-DM1.
Adverse events in the clinical trial results
In the DESTINY-Breast01 trial, incidence of at least one type of adverse event (AE) was 99.5%, and incidence of grade 3 or higher AEs was 57.1%.4 Grade 3 or higher AEs identified in more than 5% of patients were neutropenia 20.7%, anaemia 8.7%, nausea 7.6%, leucopenia 6.5%, lymphopenia 6.5% and fatigue 6.0%. The rates of the reduction and discontinuation of treatment due to AEs were 23.4% and 15.2%, respectively.
In the DESTINY-Breast02 trial, grade 3 or higher AEs were seen in 41.3% of patients treated with T-DXd . The most commonly encountered AEs were neutropenia (10.6%), anaemia (7.9%) and nausea (6.7%).7
In the DESTINY-Breast03 trial, incidence of AEs of all grades was 98.1% in the T-DXd group and 87.4% in the T-DM1 group, and the incidence of grade 3/4 AEs was 47.1% and 42.1%, respectively.9 The most common grade 3 or higher AEs for T-DXd were neutropenia (16.0%), anaemia (9.3%), thrombocytopenia (7.8%) and nausea (7.0%).
T-DXd was initially considered to be a moderately emetogenic agent because nausea occurred in more than 70% of all grades of AE in DESTINY-Breast 01, 02 and 03 studies, and the prophylactic administration of antiemetic agents was recommended.10 However, in the 2023 version 1 of the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®), the category for T-DXd was changed to a high emetic risk level.11
The AE of greatest concern for T-DXd was interstitial pneumonia, which at the beginning of the DESTINY-Breast01 trial was associated with 13.6% of interstitial lung disease (ILD) occurrences.4 Grades 1 and 2 accounted for 10.9% of AEs, grade 3 for 0.5%, and 2.2% of deaths were attributed to ILD. Initially, it was thought that ILD cases were not likely to occur after administration of T-DXd, since ILD cases occurred with a certain frequency over a 12-month period. Subsequently, chest imaging and other clinical data for 245 patients with HER2-positive advanced breast cancer who received T-DXd monotherapy (5.4 mg/kg, every 3 weeks) in two phase I trials (DS8201-A-J101, T-DXD-A104)3,12 and the phase II DESTINY-Breast01 trial were independently judged in a committee–reviewed retrospective analysis and reported at the 2021 European Society for Medical Oncology Breast Cancer Virtual Congress.13 The median age was 56 years (range 28–96), and 23.7% were Japanese; 42.4% of patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 1, and the median number of prior treatments was 7.0 (range 2–27). The median duration of treatment was 9.7 months (range 0.7–40.3). The analysis determined that 38 patients (15.5%) had drug-related ILDs. In all, 30 (78.9%) of the patients with ILDs had grade 1/2 ILDs. Grades 3 and 4 ILDs occurred in 1 (0.4%) patient each, and grade 5 occurred in 6/245 (2.4%). Of the 38 patients with ILD, 37 (97.4%) developed ILD within 12 months after treatment initiation. Of the 42% of patients who received T- DXd for more than 12 months, few had onset of ILD after 12 months on T- DXd treatment. Subsequently, a pooled analysis of nine T-DXd single-agent trials, including gastric, colorectal, and lung cancers, was conducted to evaluate ILD risk in 1150 heavily pretreated patients. Overall incidence of adjudicated T-DXd-related ILD was 15.4% (grade 1 or 2, 77.4%; grade 5, 2.2%) and within 12 months of their first T-DXd dose, 87.0% of ILD patients had their first event (median, 5.4).14 The risk of ILD was higher within the first 12 months of T-DXd administration.
In the DESTINY-Breast02 trial, the adjudication committee determined that 10.4% of patients had ILD; most of these had low–grade events, but two had grade 5 (death). In the DESTINY-Breast03 trial, 15.2% of patients had ILD, with two at grade 3 and none at grade 4 or grade 5 (death).9 The DESTINY-Breast02 and 03 trials, which enrolled patients with fewer prior treatments than those enrolled in the DESTINY-Breast01 trial, showed a lower incidence of ILD than the DESTINY-Breast01 trial. Further follow-up is needed to identify whether this difference in ILD incidence can be attributable to differences in patient background.
Quality of life evaluation
In the DESTINY-Breast03 trial, patient-reported outcomes were assessed on days 1, 2 and 3 of cycle 1 and then every two cycles until the end of treatment, then at the end of treatment, at 40–day follow-up and every 3 months thereafter until the conclusion of follow-up15 From the medical records, hospitalization-related endpoints were assessed as time to first hospitalization and length of hospital stay. Health-related quality of life according to patient-reported outcomes was assessed using the European Organization for Research and Treatment of Cancer (EORTC) cancer-specific quality of life questionnaire (QLQ-C30) global health status, and functional (physical, role, emotional, cognitive and social) and symptom scales (pain), as well as the EORTIC QLQ-BR for patients with breast cancer. Analysis involved change from baseline and time from random assignment to deterioration with no recovery (TTD) for the EORTIC QLQ-BR45 symptom scale (arm, breast) and for the EuroQol 5- dimension 5-level (EQ-5D-5L) visual analogue scale (VAS). At data cutoff, 132 patients (51.4%) in the T-DXd group and 47 (18.0%) in the T-DM1 group were receiving treatment, involving a median duration of 14.3 months (range 0.7–29.8) and 6.9 months (range 0.7–25.1), respectively.
No difference was seen in baseline global health status scores on the QLQ-C30 between the T-DXd (252 patients) and T-DM1 (259 patients) groups, and both groups generally maintained their scores until fewer than 20 patients were on treatment. At the end of treatment, change from baseline was not clinically meaningful (10 points) in either group, with a mean change from baseline score of 1.98 (standard deviation 21.34) in the T-DXd group and 4.07 (standard deviation 22.05) in the T-DM1 group. The median TTD of QLQ-C30 global health status was 9.7 months in the T-DXd group (95% Cl: 7.3–12.5) and 8.3 months in the T-DM1 group (95% CI: 7.0–10.3), with a hazard ratio (HR) of 0.88 (95% CI: 0.70–1.11; pp=0.283). The TTD for all prespecified QLQ–C30 subscales was longer in the T-DXd group; for example, pain symptoms showed an HR of 0.75 (95% CI: 0.59–0.95; pp=0.0146), and the affective scale exhibited an HR of 0.69 (95% CI: 0.53–0.89; p=0. 005).
The TTD for the EQ-5D-5L VAS was significantly longer in the T-DXd group, with a median of 13.2 months (95% CI: 10.1–15.3) in the T-DXd group and 8.5 months (95% CI: 7.3–10.4) in the T-DM1 group, with an HR of 0.77 (95% CI: 0.61–0.98; p=0. 0.035). In addition, 18 patients (6.9%) in the T-DXd group and 19 (7.2%) in the T-DM1 group required hospitalization.
Clinical markers and biomarkers
Subanalysis of the DESTINY-Breast01 trial showed that the efficacy of T-DXd did not correlate with that of pretreatment T-DM1 and use of pertuzumab.14 The adjusted ERBB2 plasma copy number and the HER2 extracellular domain values at baseline did not predict clinical outcomes. Neither PIK3CA mutations seen in circulating tumour DNA at baseline nor the presence of PIK3CA and ERBB2 gain-of-function mutations were associated with clinical outcomes in DESTINY-Breast01. Several somatic alterations were seen in single genes that were associated with PFS and ORR, but no firm markers were established that could be used in routine practice.
In the DESTINY-Breast01 trial, the pre- and post-treatment tumour DNA changes identified in approximately 30 patients showed that the genetic changes acquired in DNA damage repair genes, such as BRCA1, ATM and POLE, may be associated with resistance.14
Effects on brain metastases in patients with HER2-positive metastatic breast cancer
Subgroup analysis of patients with central nervous system (CNS) metastases at baseline in the DESTINY-Breast01 trial has been reported.16 At baseline, 24 of 184 patients (13.0%) had CNS metastases, and these patients were in better overall health than the total population (62.5% with ECOG PS 0 for CNS metastases, versus 55.4% for the total population), and they had more hormone receptor–negative results (58.3% versus 45.1%). There were no other major differences in background. In patients with CNS metastases, ORR was 58.3% (95% CI: 36.6–77.9) and disease control rate was 91.7%, including 4.2% CR, 54.2% PR and 33.3% stable disease. Median PFS was 18.1 months (95% CI: 6.7–18.1). Among those with CNS metastases at baseline, two patients (8.3%) showed progression in the brain. Among those without CNS metastases at baseline, two (8.3%) showed progression in the brain. This progression occurred on days 78 and 85, respectively, in the two patients with CNS metastases at baseline, and on days 323 and 498, respectively, in those without CNS metastases at baseline. As patients without CNS metastases at baseline did not undergo routine brain examination, it is possible that brain metastasis (BM) had already been present when the disease progressed at other sites but was not clinically problematic, which suggests that T-DXd suppressed the symptoms of BM.
An exploratory analysis of the DESTINY-Breast03 trial found that ORR in patients with BM at baseline was 67.4% in the T-DXd group versus 20.5% in the T-DM1 group, and the median PFS was 15.0 months in the T-DXd group (95% CI: 12.5–22.2) versus 3.0 months (95% CI: 2.8–5.8) in the T-DM1 group (HR: 0.25 [95% CI: 0.13–0.45]).17 The ORR in patients without BM was 82.1% versus 36.6%, and the median PFS was NE (95% CI: 22.2–NE) versus 7.1 months (95% CI: 5.6–9.7) (HR: 0.30 [95% CI: 0.22–0.40]). No difference in efficacy was observed between patients with and without BM at baseline.In patients with BM at baseline at enrollment, response rates for intracranial lesions were CR 27.8% and PR 36.1% in the T-DXd group and CR 2.8% and PR 30.6% in the T-DM1 group
Patients in both the DESTINY-Breast01 and DESTINY-Breast03 trials were ineligible for cases of active BM, and all results concern patients with inactive BM.
Patients in both DESTINY-Breast01 and DESTINY-Breast03 were ineligible if they had untreated, symptomatic BM or had been treated for BM within 2 weeks (60 days in DESTINY-Breast01) prior to enrollment. Therefore, all of the above results are for patients with asymptomatic or post-treatment BM.18 The TUXEDO-1 trial included adult patients with newly diagnosed HER2-positive breast cancer who had untreated BM and had previously received trastuzumab and pertuzumab, or patients with BM that worsened after local treatment and was not amenable to rapid local therapy. Patients received T-DXd 5.4 mg/kg every 3 weeks. The primary endpoint was intracranial response rate, as determined centrally using Response Assessment in Neuro-Oncology BM criteria, which is an efficacy index for brain tumours.19 Secondary endpoints included the clinical benefit rate, extracranial response rate, PFS, OS, safety and quality of life. Following Simon’s two-stage design, 15 patients were enrolled with 60% as the threshold for a clinically meaningful response rate and 26% as the threshold for a non-clinically meaningful response rate. A response in at least seven patients was required to be considered valid. In all, 40% had neurological symptoms at baseline; 60% had worsening BM following local therapy; 60% had received T-DM1; and median number of prior treatments for metastatic breast cancer prior to T-DXd was 2 (range 1–5).
The results showed an intracranial response rate of 73.3% (in 11 of 15 patients) in the intention–to–treat analysis and 78.6% (11 of 14 patients) in the per–protocol analysis, from which one patient was excluded due to dural metastasis. Tumour shrinkage was observed in all patients. Median follow-up was 11 months (range 3–17), and median PFS was 14 months (95% CI: 11.0–not recorded). The clinical benefit rate was 86.7% in the intention-to-treat analysis, and median OS had not yet been reached. Response was seen in 5 of 8 patients (62.5%) who had measurable extracranial disease at baseline.
The DEBBRAH Trial Is a five-cohort Phase II study of patients with pretreated HER2-positive or HER2-low advanced breast cancer with stable, untreated, or progressive BM and/or leptomeningeal carcinomatosis.20 Among patients with HER2-positive advanced breast cancer, the results for patients with non-progressive BM after local therapy (eight patients: Cohort 1), asymptomatic untreated BM (four patients: Cohort 2), and progressive BM after local therapy (nine patients: Cohort 3) were enrolled. The primary endpoints were PFS at 16 weeks in cohort 1 and overall intracranial response rate (ORR-IC) in cohorts 2 and 3. In all, 21 patients received T-DXd. In cohort 1, the 16 week PFS rate was 87.5%; the ORR-IC was 50.0% in cohort 2, and it was 44.4% in cohort 3. Overall, ORR-IC in patients with active BM was 46.2%. In patients with measurable intracranial or extracranial lesions at baseline, ORR across the three cohorts was 66.7% 80.0% (95% CI: 28.4–99.5) in cohort 1, 50.0%(95% CI: 6.7-93.2) in cohort 2 and 66.7% (95% CI: 29.9-92.5) in cohort 3. Furthermore, PRs were observed in all patients. Both the TUXEDO-1 and DEBBRAH trials showed promising results for T-DXd in untreated or active BM.
In the HER2CLIMB study, the median intracranial progression-free survival in HER2-positive breast cancer patients with BM was 9.9 months in the tucatinib, trastuzumab, and capecitabine group versus 4.2 months in the placebo, trastuzumab, and capecitabine group.21 The median OS in patients with BM was reported as 21.6 months in the tucatinib combination arm versus 12.5 months in the placebo arm. In patients with active BM, the response rate for tucatinib in combination with trastuzumab and capecitabine was reported to be 47.3% with 26 of 55 patients having an intracranial response. 21 The efficacy of tucatinib in patients with BM has been demonstrated. A phase II study (HER2CLIMB-04) to evaluate the combination of T-DXd and tucatinib is currently under way.22
Ongoing trials
Following the favourable OOR and PFS of the DESTINY-Breast01 and DESTINY-Breast03 trials, the DESTINY-Breast09 trial is currently being conducted in the first-line setting in 1,134 patients with HER2-positive unresectable and/or metastatic breast cancer.23 Patients are randomized 1:1:1 to T-DXd + pertuzumab or T-DXd + placebo with trastuzumab-pertuzumab and taxane as standard therapy (Table 1).22–27
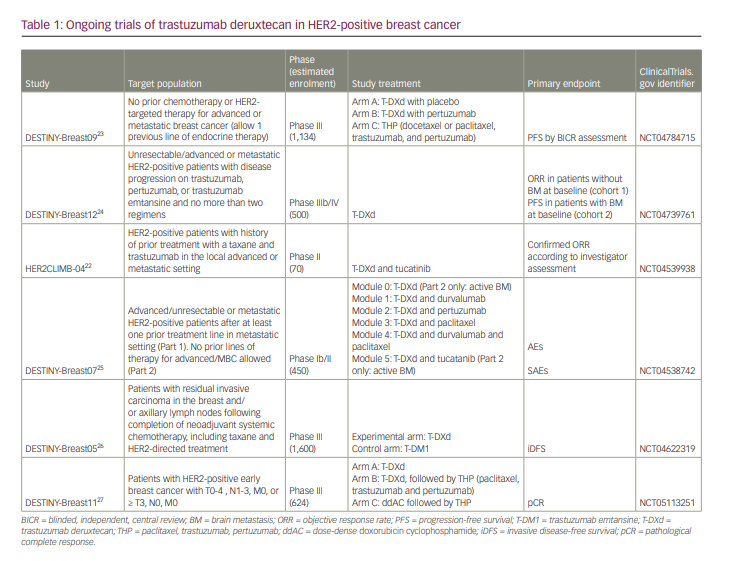
The DESTINY-Breast12 trial, which is a phase IIIb/IV trial conducted to investigate the occurrence of BM during T-DXd use and examine T-DXd efficacy against BM, is also ongoing.24 This study is evaluating the efficacy of T-DXd in 500 patients with no BM at baseline (cohort 1) and those with BM (cohort 2) who have received two or fewer prior regimens of any anti-HER2 agents, including in perioperative treatment. The primary endpoints are the ORR in Cohort 1 and PFS in Cohort 2.
DESTINY-Breast07, a phase I/II trial, is currently being conducted to investigate the combination of T-DXd.25 The study compares T-DXd, T-DXd + durvalumab, T-DXd + pertuzumab, T-DXd + paclitaxel, T-DXd + durvalumab + paclitaxel, and T-DXd + tucatinib in 450 patients with HER2-positive breast cancer without a history of treatment for metastatic recurrent breast cancer. The primary endpoints are AEs and serious AEs. One regimen is allowed in Part 2 for patients with active BM.
The DESTINY-Breast0526 and DESTINY-Breast1127 trials are being conducted in a perioperative setting. The DESTINY-Breast05 trial assigns 1:1 to T-DM1 and T-DXd in 1,600 patients who had received six or more cycles of preoperative chemotherapy and either had residual lymph node metastases or were had stage III before treatment and did not achieve pathological complete response.26 Stratification factors include preoperative status, hormone receptor status, postoperative lymph node pathology (ypN1-3 versus ypN0) and preoperative anti-HER2 drug (one versus two drugs). The primary efficacy endpoint is invasive disease-free survival established by investigator assessment. The secondary endpoints are OS and disease-free survival.
DESTINY-Breast11 is a Phase III, open-label study comparing T-DXd monotherapy or T-DXd followed by THP (paclitaxel, trastuzumab and pertuzumab) with dose-dense AC(doxorubicin cyclophosphamide)-THP as preoperative therapy in patients with high-risk HER2-positive early breast cancer.27 This study targets 624 patients with HER2-positive early-stage breast cancer and lymph node metastasis or T3 or greater. The primary endpoints are pathological complete response rate, and the secondary endpoints include event-free survival, invasive disease-free survival and OS.
Conclusions
In the treatment of HER2-positive advanced recurrent breast cancer, T-DXd showed significant PFS and OS prolongation in the DESTINY-Breast02 study, which compared TPC (trastuzumab + capecitabine and lapatinib + capecitabine) in previously treated patients with T-DM1. The DESTINY-Breast03 trial, comparing T-DXd with T-DM1, also showed significant PFS and OS prolongation, leading to recommendation for use in second-line therapy.
Reported grade 3 or higher AEs include nausea and fatigue, as well as haematological toxicities such as anaemia and neutropenia. Nausea was seen in more than 70% of cases when all grades were included, and a prophylactic administration of antiemetic agents is recommended. Recent reports have indicated that interstitial pneumonia occurs more frequently within the first 12 months of treatment.
There are several reports of T-DXd efficacy for BM, but there are no prospective comparative studies on its use in active BM.
Many clinical trials using T-DXd are ongoing, and these require further attention.