Bladder cancer (BC) is the sixth most common cancer in the world, accounting for 81,180 estimated new cases and 17,100 deaths in 2022.1 BC is most frequently diagnosed among people aged 65–74 years.1 For patients with disease limited to the bladder, the depth of invasion of the primary tumour is the most important prognostic variable in determining the risk of recurrence or progression. Approximately 70% of patients present with non-muscle-invasive bladder cancer (NMIBC), including carcinoma in situ or exophytic tumours that are confined to the mucosa or lamina propria. Tumours that invade the detrusor muscle, or muscle-invasive bladder cancer (MIBC), constitute 20–25% of cases and have a higher propensity for rapid growth and distant metastasis. For a patient with an initial diagnosis of NMIBC, 15–20% of cases progress to MIBC.2 Since the 1970s, the 5-year overall survival (OS) rate for those diagnosed with BC in the USA has improved to 77.1%.1 However, the 5-year OS rate for patients with MIBC is approximately 60–70%.2 Once metastatic, BC is associated with a 5-year survival rate of only 5–30%.3
Cisplatin-based neoadjuvant chemotherapy (NAC) has been explored since the 1980s as a treatment strategy for MIBC with potential benefits of downstaging the primary tumour and eliminating micro-metastatic disease prior to radical cystectomy (RC) and pelvic lymph node dissection.4,5 The survival benefit of NAC appears to be strongly related to downstaging the tumour; patients with a pathological complete response, or pT0, have a 5-year OS rate of 85%,6 and those with downstaging to non-muscle-invasive disease, or <pT2, have a 5-year OS rate of 83.6%.7 However, BC is a geriatric disease. Many patients are ineligible for cisplatin-based treatment based on meeting one of the following criteria: Eastern Cooperative Oncology Group Performance Status Scale score ≥2, renal insufficiency, grade ≥2 hearing loss, grade ≥2 neuropathy and/or New York Heart Association class III heart failure.8 In these patients, or in those ineligible for or refusing cystectomy, there is no single standard of care. Trimodality therapy (TMT) is a widely used treatment strategy that incorporates maximal transurethral resection of the bladder tumour (TURBT), radiation therapy and concurrent chemotherapy. In multiple prospective clinical trials and large institutional series, TMT has demonstrated 5-year OS rates of 48–65%, comparable to survival outcomes seen with NAC followed by RC.9
Over the past decade, a variety of novel therapies, most notably immune checkpoint inhibitors (ICIs), have revolutionized the management of BC. This review aims to describe the evolution and future directions of the treatment paradigm in non-metastatic MIBC in the context of these new agents.
Rationale and current strategies for bladder preservation
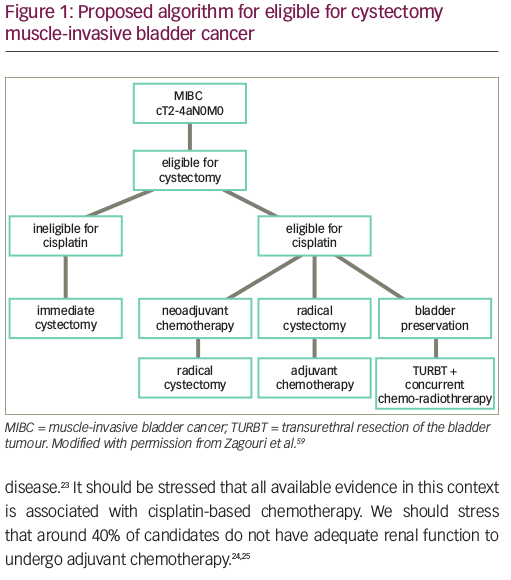
Despite the efficacy of NAC followed by cystectomy, and the advances in adjuvant therapy in improving outcomes for MIBC, these are not an ideal strategies for many patients. Many patients are unfit for RC, ineligible for cisplatin-based chemotherapy, or unwilling to undergo RC due to concerns over morbidity and urinary diversion. Indeed, RC results in high post-operative complication rates and significant mortality of 1.5% in some population studies.10,11 Considering that the median age of diagnosis of BC is 73 years, and older age is associated with higher rates of postoperative complications and perioperative mortality, there is good reason to offer bladder preservation treatments to patients.1,12
Partial cystectomy and TMT consisting of TURBT + concurrent chemoradiation are currently the main approaches for bladder preservation. Partial cystectomy is reserved for selected patients, driven by anatomical location, solitary versus multifocal form, and pathology of the tumour.13 However, its overuse may lead to undertreatment and higher associated mortality.14 TMT is supported by multiple prospective and retrospective studies, demonstrating safety and efficacy.15–17 The seminal BC2001 study evaluated concurrent chemoradiation versus radiotherapy alone, and demonstrated a 5-year OS rate of 48% in the chemoradiation group versus 35% in the radiotherapy-alone group.15 A more recent meta-analysis of several TMT clinical trials demonstrated a 56% 5-year OS rate with TMT.16 In addition, a pooled analysis of multicentre, prospective, Radiation Therapy Oncology Group bladder-preserving protocols showed that the 5-year OS rates was 57% for patients who underwent bladder preservation with cystectomy reserved as a salvage option.17 This is comparable to RC and its 5-year OS rates of 49–57%.18 In a more recent multi-institutional, retrospective, propensity-matched analysis of 703 patients, there was no significant difference between RC and TMT cohorts in metastasis-free, distant failure-free and pelvic nodal failure-free survival at 5 years.19 In fact, cancer-specific survival and OS favoured the TMT patient cohort over the RC patient cohort.19 Thus, despite the absence of randomized data, TMT is a viable alternative to NAC + RC in the treatment of selected MIBC.
Novel approaches to bladder preservation
The excellent outcomes of patients who achieve pathological downstaging to non-muscle-invasive disease (<pT2), especially those who achieve a pathological complete response (pCR), has led many to ask whether RC is even necessary in this subgroup. Recently, there have been efforts to study bladder preservation with active surveillance or non-surgical local therapy instead of RC in patients who received NAC and achieved clinical ypT0 on restaging TURBT. With the advent of tumour genomic profiling technology, risk stratification and prognosis modelling have started to identify biomarkers that would help predict response to NAC and ultimately provide treatment guidelines after NAC. Plimack et al. analysed various genomic biomarkers in 34 patients who were treated with three cycles of NAC.20 Of the 34 patients identified, 15 were found to have excellent pathological response (defined as ≤ypT1). When compared with non-responders, 87% of the patients with pathological response were found to have alterations in the genes ataxia telangiectasia mutated (ATM), RB transcriptional corepressor 1 (RB1) and FA complementation group C (FANCC).20 A subsequent study found that in a 48-patient cohort, 10 patients had genetic alterations in the ERRC2 gene. Of these 10 patients, eight responded to NAC (defined as pT0/pTis/pTa at cystectomy).21 Iyer et al. examined the role of ATM, RB1, FANCC and ERRC2 alterations in a prospective neoadjuvant trial of dose-dense gemcitabine plus cisplatin.22 Overall, nine patients were identified as having alterations in the specified genes and eight of those nine patients were found to have pathological downstaging of disease at cystectomy. Interestingly, all tumours with these gene alterations were associated with higher tumour mutational burden.22
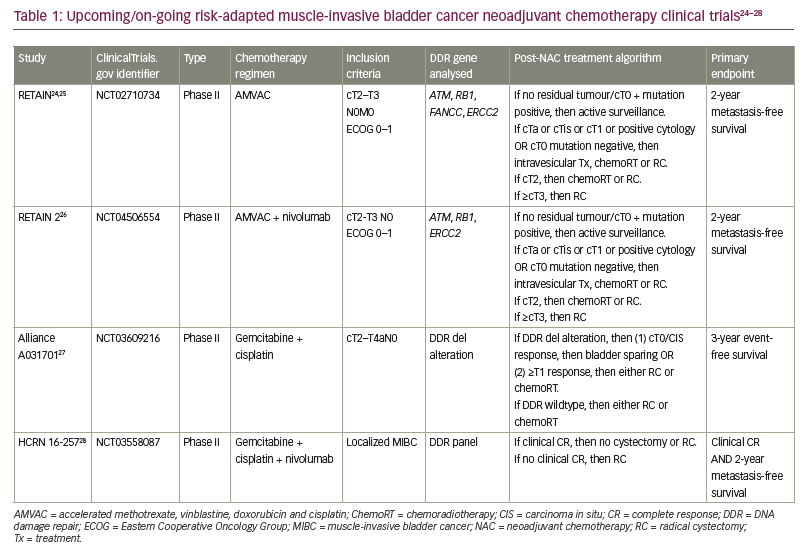
Several clinical trials have now begun to incorporate these biomarkers to identify which patients may be suitable for active surveillance after NAC rather than proceeding with definitive local radiation therapy and RC.23 The neoadjuvant regimen, key inclusion criteria, post-NAC treatment algorithm, genetic sequencing analysed and primary endpoint for each clinical trial are summarized in Table 1.24–28 First, the RETAIN trial is a phase II clinical trial that explores a risk-adapted approach to subsequent bladder therapy in MIBC based on pathological response to NAC and genetic tumour biomarkers (ClinicalTrials.gov identifier: NCT02710734).24 The RETAIN trial is different from previous clinical trials and standard of care as it offers bladder-sparing options in patients who are found to have pathological downstaging to NAC. Patients with pCR to NAC and found to have mutations in ATM, RB1, FANCC or ERCC2 are placed on active surveillance. The primary endpoint is metastasis-free survival. Data from an interim analysis of RETAIN presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium 2021 showed that 89% of the patients in the active surveillance group had successful bladder preservation at a median follow-up of 14.9 months.25 RETAIN 2 is similarly designed to RETAIN, with the addition of nivolumab to the NAC regimen (ClinicalTrials.gov identifier: NCT04506554).26 Third, the Alliance Cooperative Group study A031701 (ClinicalTrials.gov identifier: NCT03609216)27 uses a NAC regimen of dose-dense gemcitabine with cisplatin and a possible bladder-sparing pathway to those with mutations in the DNA damage response and repair genes and <cT1 disease on restaging TURBT. Finally, HCRN 16-257 offers bladder-sparing options that are similar to the previously described three trials, adding nivolumab to gemcitabine + cisplatin as the NAC regimen (ClinicalTrials.gov identifier: NCT03558087).28
New treatments in the neoadjuvant setting
Since the seminal Southwest Oncology Group (SWOG) 8710 study demonstrated an OS advantage of neoadjuvant therapy with methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) over RC alone, cisplatin-based NAC has been the standard of care.6 Regimens such as dose-dense MVAC (ddMVAC) and cisplatin-gemcitabine have been studied in a prospective fashion and replaced traditional MVAC.29,30 Two recent randomized studies have evaluated cisplatin-gemcitabine and ddMVAC in the neoadjuvant setting. The VESPER trial was a randomized phase III trial that compared the efficacy of six cycles of ddMVAC with that of four cycles of cisplatin-gemcitabine in MIBC in the perioperative setting.31 Of note, the study included both neoadjuvant and adjuvant therapy groups. This study demonstrated pCR (pT0) rates of 42% in the neoadjuvant ddMVAC cohort (n=218) versus 36% in the neoadjuvant cisplatin-gemcitabine cohort (n=219).31 The primary analysis of the study found ddMVAC was associated with an improved 3-year progression-free survival compared with cisplatin-gemcitabine (64% versus 56%; hazard ratio [HR] 0.77, 95% confidence interval [CI] 0.57–1.02; p=0.66); however, this did not meet the prespecified threshold for statistical significance.32 Notably, this analysis included patients treated in the adjuvant setting. When comparing only NAC patients (n=437), 3-year progression-free survival was significantly higher with ddMVAC compared with cisplatin-gemcitabine (66% versus 56%; HR 0.70, 95% CI 0.51–0.96; p=0.025). Another recent study, SWOG S1314 (ClinicalTrials.gov identifier: NCT02177695), a randomized phase II study, showed pT0 rates of 28% in the ddMVAC group versus 30% in the cisplatin-gemcitabine group but was not powered to detect outcome differences between the two arms.33 Thus, despite the VESPER data suggesting improved activity with ddMVAC compared with cisplatin-gemcitabine, both neoadjuvant regimens remain acceptable standards of care in cisplatin-eligible patients.
Neoadjuvant immune checkpoint inhibition
Not all patients are eligible for cisplatin-based therapy, and multiple new agents have entered the BC treatment landscape. The activity of ICIs in the metastatic setting has led to great interest in exploring their use in the neoadjuvant setting. PURE-01 was a non-randomized, phase II study of neoadjuvant pembrolizumab in MIBC (Table 2).34–36 The patient population (n=50) comprised 42% with T2 disease, 54% with T3 disease and 4% with N1 disease. With RC intended as definitive treatment, patients were enrolled regardless of cisplatin eligibility, and programmed death-ligand 1 (PD-L1) positivity was defined as ≥10% by combined positive score (CPS) using the 22c3 assay. Pembrolizumab 200 mg was administered for three cycles every 3 weeks and patients underwent RC within 1–3 weeks after their last dose. The primary endpoint was pCR, which was defined as pT0 at RC. Interestingly, overall, 42% of patients achieved pCR. In those with a PD-L1 CPS ≥10%, the pCR rate was 54%.34 The most common side effect was thyroid dysfunction, occurring in about 18% of the patients, and only 6% of patients had grade ≥3 adverse events (AEs). Only one patient discontinued pembrolizumab and switched to cisplatin-based chemotherapy, and all treated patients underwent RC.34
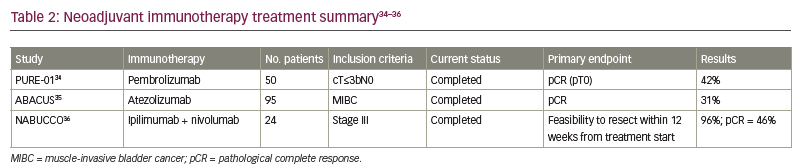
The ABACUS trial was another phase II non-randomized study but of neoadjuvant atezolizumab in MIBC.35 A total of 95 patients were enrolled – 74% with T2 disease, 18% with T3 disease and 8% with T4 disease; no patient had node-positive disease.35 pCR was the primary endpoint. Eligibility criteria included patients who were cisplatin ineligible or patients who refused cisplatin-based NAC. Of the 95 patients, 87 patients (92%) underwent cystectomy. PD-L1 positivity was defined as ≥5% using the SP142 assay. Of the 88 patients who were assessed for the primary endpoint, 35 (40%) were PD-L1 positive. Prior to RC, patients received atezolizumab 1,200 mg every 3 weeks for two cycles. Median time from start of atezolizumab to surgery was 5.6 weeks. The study demonstrated a pCR rate of 31% in the overall population and a pCR rate of 37% in patients who were PD-L1 positive. Grade 3 and 4 AEs were reported in 11% of patients, and three patients who received atezolizumab were unable to undergo RC due to reduced performance status, myocardial infarction and pneumonia.35
The NABUCCO trial examined ipilimumab plus nivolumab as a combination immunotherapy in the neoadjuvant setting for locoregionally advanced BC.36 This was a single-arm feasibility trial that enrolled 24 patients with stage III urothelial cancer (UC), with 58% of patients with T3–T4a disease without nodal involvement and 42% with N1–N3 disease. PD-L1 positivity was defined as a CPS score of >10%, and 63% of patients were PD-L1 positive. In addition, patients were either cisplatin ineligible or refused cisplatin-based chemotherapy. All patients underwent RC. Prior to surgery, patients were given ipilimumab 3 mg/kg on Day 1, then ipilimumab 3 mg/kg plus nivolumab 1 mg/kg on Day 22, and then nivolumab 3 mg/kg on Day 43. Overall, 55% of patients reported grade 3–4 immune-related AEs. Only one patient had to delay RC due to AEs, but 23 patients (96%) underwent RC within 12 weeks of finishing immunotherapy. The primary endpoint was feasibility to resect within 12 weeks from first infusion, and pCR was evaluated as a secondary endpoint. Overall, 11 patients (46%) had a pCR and 14 patients (58%) had no residual invasive cancer, which was defined as either pCR or small carcinoma in situ/pTa focus. Furthermore, the pCR rate in patients with PD-L1 CPS score of >10% was 73%.36
The high pCR rates observed in these studies has led to considerable interest in ICI therapy in the neoadjuvant setting. Several on-going phase III trials are exploring the use of neoadjuvant ICIs and will provide critical data on how ICI monotherapy may be employed in the neoadjuvant setting.
Combining neoadjuvant immune checkpoint inhibition with other therapies
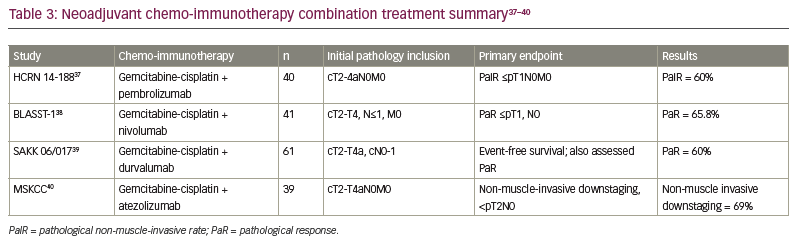
Given the high pCR rates seen with ICIs alone and the known efficacy of platinum chemotherapy, several studies have explored the synergy of ICIs with standard chemotherapy. Various ICIs in combination with standard cisplatin-based chemotherapy in the neoadjuvant setting have been evaluated (Table 3).37–40 Due to the non-randomized nature of these trials and the hazards of cross-trial comparisons, it remains unclear how much is gained by the addition of ICI to chemotherapy in the neoadjuvant setting. However, the combination does appear to be safe and allow the majority of patients to proceed to curative-intent cystectomy, although the challenge of finding a suitable neoadjuvant regimen remains for patients with MIBC who are not eligible for cisplatin.
Other treatments, in particular enfortumab vedotin, have been explored as possible options for neoadjuvant therapy for MIBC. Enfortumab vedotin, an antibody–drug conjugate, has established a role in patients with locally advanced or metastatic UC who have received prior treatments with programmed cell death protein 1 (PD-1) or PD-L1 inhibitors and who are cisplatin ineligible.41 A randomized phase III trial comparing enfortumab vedotin plus pembrolizumab with combination gemcitabine-cisplatin (Keynote-B15/EV304) is exploring the efficacy of this combination in the neoadjuvant setting for MIBC, with pCR and event-free survival as co-primary endpoints.42 Other combination trials include durvalumab with oleclumab (anti-CD73) (ClinicalTrials.gov identifier: NCT03773666),43 pembrolizumab with abemaciclib (cyclin-dependent kinase 4/6 inhibitor) (ClinicalTrials.gov identifier: NCT03837821)44 and pembrolizumab with epacadostat (ClinicalTrials.gov identifier: NCT03832673).45 The clinical value of combination immuno-oncology with other therapies remains to be seen. However, observing the diversifying landscape of clinical trials exploring these different options offers hope that the future holds neoadjuvant strategies beyond the current cisplatin-based treatment.34–36
Adjuvant chemotherapy in muscle-invasive bladder cancer
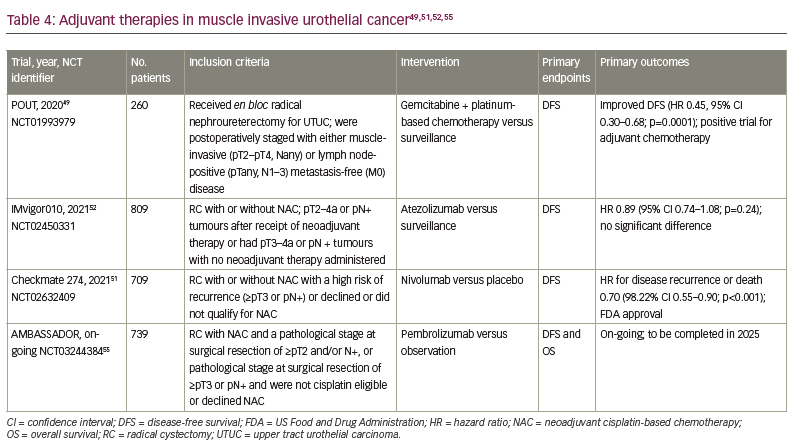
Patients with completely resected, high-risk UC (defined as residual cancer ≥pT2 or pN+ after NAC chemotherapy) have a 5-year survival rate of only 60%.46 For patients who undergo RC, are at high risk for recurrence based on pathological findings and have not received NAC, adjuvant chemotherapy is indicated. Several prospective trials have suggested survival benefits for adjuvant cisplatin-based therapies but were limited by sluggish accrual and thus inadequate power.47,48
Adjuvant therapy in upper tract urothelial carcinoma
Though not strictly germane to BC, the recently reported POUT trial may offer some insight into adjuvant therapy in UC.49,50 The POUT adjuvant study enrolled patients with upper tract UC (UTUC) who underwent radical nephroureterectomy and were postoperatively staged with either muscle-invasive (≥pT2) and/or lymph node-positive disease without evidence of metastatic disease. A total of 261 patients were randomized 1:1 to receive either platinum-doublet chemotherapy (with cisplatin-gemcitabine or carboplatin-gemcitabine) or surveillance. Approximately 60% of patients in the adjuvant chemotherapy arm received gemcitabine-cisplatin and 40% received gemcitabine-carboplatin. The adjuvant therapy group had significantly improved disease-free survival (DFS; HR 0.51, 95% CI 0.35–0.76; p=0.0006) and metastasis-free survival (HR 0.52, 95% CI 0.36–0.77; p=0.0007). As this study was specific to UTUC, the data should not be applied to MIBC. The role of carboplatin-based adjuvant chemotherapy in BC is not established and further studies that explore this regimen need to be conducted prior to its application.
Adjuvant immune checkpoint inhibition in muscle-invasive bladder cancer
Checkmate 274: Adjuvant nivolumab
The Checkmate 274 trial was a phase III trial that evaluated adjuvant nivolumab compared with placebo.51 The trial included patients who underwent RC with or without NAC. Approximately 21% of patients in each arm had an upper tract primary tumour. Eligible patients had evidence of disease with a high risk of recurrence, defined as ≥pT3 or pN+ if NAC was received or ≥pT2 if no NAC was received. Patients were stratified according to tumour PD-L1 expression level (≥1% versus <1% or indeterminate), pathological nodal status and receipt of NAC. Previous NAC was administered in approximately 43% of both groups.51 The primary endpoints were DFS among all the patients (intention-to-treat population) and among patients with a tumour PD-L1 expression level of 1% or more as measured by the pharmDx 28-8 PD-1 immunohistochemical assay. Secondary endpoints included survival free from recurrence outside the urothelial tract, OS and disease-specific survival in both populations. A total of 709 patients received nivolumab 240 mg or placebo every 2 weeks until evidence of disease recurrence up to 1 year. The percentage of patients with DFS at 6 months was 74.9% with nivolumab and 60.3% with placebo (HR 0.70, 95% CI 0.55–0.90; p<0.001). The percentage of patients with disease-free recurrence outside the urothelial tract at 6 months was 77.0% with nivolumab and 62.7% with placebo (HR 0.72, 95% CI 0.59–0.89). DFS in patients with PD-L1 expression of 1% or more was also higher in the nivolumab group. Treatment-related adverse events (TRAEs) of any grade that led to discontinuation of the trial regimen occurred in 12.8% of the patients in the nivolumab group and 2.0% of those in the placebo group. Grade 3 or higher AEs occurred in 42.7% and 36.8%, respectively. Prior to this study, no previous adjuvant systemic therapies have been shown in a randomized phase III study to improve outcomes in patients with BC who are not eligible for cisplatin-based NAC or in those with pathological evidence of residual disease despite NAC. This study led to the first US Food and Drug Administration approval of a ICI for adjuvant treatment of patients with high-risk UC in August 2021.51 Notably, the European Medicines Agency has restricted its approval to those patients with PD-L1 >1%. One limitation of the study is that OS data were immature at the time of analysis. An OS benefit is the gold standard in the adjuvant setting, but a DFS improvement in UC is in itself clinically meaningful. Additionally, DFS has proven to be a good surrogate for OS in this setting for UC. As the first approved adjuvant therapy for BC, this represents a watershed moment for the perioperative treatment of BC.
IMvigor010: Adjuvant atezolizumab
This phase III multicentre study was developed to evaluate adjuvant treatment with atezolizumab compared with observation in patients who were at high risk for recurrence following resection.52 Patients were eligible if they had pT2–4a or pN+ tumours after receipt of NAC or had pT3–4a or pN+ tumours without NAC. A total of 809 participants with MIBC or UTUC (capped at 10%) were enrolled. Previous NAC was administered in approximately half of the study population. Patients were enrolled regardless of PD-L1 status, which was measured using the Ventana SP142 immunohistochemical assay. Patients in the treatment arm received atezolizumab 1,200 mg every 3 weeks for up to 1 year as long as they did not have disease recurrence or dose-limiting toxicity. The primary endpoint was DFS in the intention-to-treat population. The median DFS with atezolizumab and observation were 19.4 months (95% CI 15.9–24.8) and 16.6 months (95% CI 11.2–24.8), respectively; the HR was 0.89 (95% CI 0.74–1.08; p=0.24). The median DFS in the PD-L1 subgroup IC0 or IC1 (defined as PD-L1 expression of <5%) was 16.4 months (95% CI 12.0–20.0) in those who received atezolizumab compared with 11.1 months (95% CI 8.3–16.6) in the observation group (HR 0.85, 95% CI 0.66–1.10). The median DFS in the PD-L1 subgroup IC2 or IC3 (defined as PD-L1 expression of >5%) was 24.8 months (95% CI 17.2 – not estimable [NE]) in those who received atezolizumab compared with 41.4 months (95% CI 17.4–NE) in the observation group (HR 1.01, 95% CI 0.76–1.35). There was also no appreciable difference in OS, although these data were immature at the time of publication. The rates of AEs of any kind were 94%, with approximately 17% grade 3 or higher AEs in the atezolizumab group. Thus, this study did not reach its efficacy endpoint in the intention-to-treat population.52
Notably, even though this trial did not reach its primary endpoint, it created an ideal setting for investigating whether patients with molecular residual disease and a high likelihood of recurrence after surgery could benefit from ICIs. A secondary analysis of 581 patients who were evaluable for circulating tumour DNA (ctDNA)53 was conducted to evaluate the impact of ctDNA post-surgery as a biomarker for outcomes. The 214 patients (37%) who were positive for ctDNA at the start of adjuvant therapy had a dramatically higher risk of relapse (HR 6.3 in the observation arm; 95% CI 4.45–8.92).53 Of note, those who were ctDNA positive had improved DFS (HR 0.58, 95% CI 0.43–0.79) and OS (HR 0.59, 95% CI 0.41–0.86) in the atezolizumab arm compared with the observation arm. In those who were negative for ctDNA, there was no difference in DFS or OS between the two groups. Additionally, the rate of ctDNA clearance at Week 6 of the adjuvant period was higher in the atezolizumab arm than in the observation arm (18% versus 4%; p=0.0204). These findings could pave an innovative direction in the use of ctDNA as a biomarker for molecular residual disease as an integral component of post-resection care in UC. Indeed, the IMvigor011 study is an on-going phase III trial of adjuvant atezolizumab versus placebo in the high-risk group of patients who are ctDNA positive following cystectomy (ClinicalTrials.gov identifier: NCT04660344).54
AMBASSADOR: Adjuvant pembrolizumab
AMBASSADOR (ClinicalTrials.gov identifier: NCT03244384) is an on-going randomized phase III trial of adjuvant pembrolizumab compared with observation in patients with high-risk MIBC or locally advanced BC post-cystectomy.55 The trial has completed enrolment of 739 patients who either received NAC and had a pathological stage at surgical resection of ≥pT2 and/or N+, or had a pathological stage at surgical resection of ≥pT3 or pN+ and were not cisplatin eligible or declined NAC. Those who received pembrolizumab will receive up to 18 cycles unless discontinuation is necessary due to adverse toxicities or disease progression. The co-primary outcomes are DFS and OS. Secondary outcomes include OS and DFS in PD-L1-positive or -negative patients. The estimated completion date for the study is in 2025.
Summary: What does the future hold for adjuvant checkpoint inhibitors?
Many questions have arisen regarding the different results of the Checkmate 274 and IMvigor010 trials.51,52 Both trials possessed similar designs but led to disparate results. A key difference between the trials was the premise of different control groups. The control group in the Checkmate 274 trial was placebo whereas the IMvigor010 trial used surveillance. The use of placebo potentially avoids the bias of informed censoring that can occur in observations groups. The early dropout rate can artificially improve the performance of a control arm. In the IMvigor010 trial,52 35 patients in the observation arm withdrew whereas only 15 withdrew from the adjuvant therapy arm. DFS rates secondary to bias of informative censoring may have been overestimated in the observation arm and underestimated in those who received atezolizumab. Other nuances between the two trials may include differences in baseline patient characteristics that were unaccounted for, such as biomarker expression (e.g. PD-L1 expression). The ctDNA analysis in IMvigor010 demonstrates the crucial role that molecular residual disease can play in the risk of relapse and response to adjuvant ICIs, so imbalances between groups and across studies could have played a significant role in the disparate outcomes.
The primary endpoints for the trials discussed above were also similar. However, Checkmate 274 and IMvigor010 focused on DFS whereas the co-primary endpoints of the on-going AMBASSADOR trial are DFS and OS.51,52,55 A study performed on patients with MIBC after RC aimed to validate the association of DFS and OS.56 The study validated the correlation between 2- and 3-year DFS and 5-year OS, but did not comment on superiority of either primary endpoint. Thus, even though Checkmate 274 was the first trial to demonstrate an improvement in a surrogate endpoint with an adjuvant ICI, the optimal strategy for incorporating adjuvant ICIs in MIBC requires further elucidation from on-going studies (Table 4).49,51,52,55
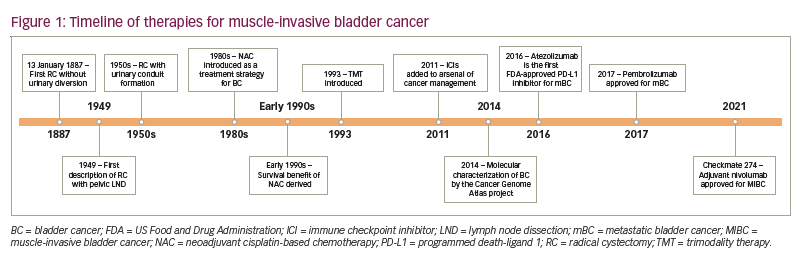
A timeline summarizing the development of therapies for MIBC is provided in Figure 1.
Conclusion
The treatment of MIBC is rapidly evolving, with frontiers in adjuvant therapy, novel neoadjuvant therapies and opportunities for bladder preservation. The recent approval of adjuvant nivolumab in MIBC is likely to be the first of many novel means of improving BC outcomes using ICIs in the perioperative setting. As further studies such as those discussed in this review are completed, we will be closer to our goal of improving the morbidity and mortality from MIBC.