Urothelial carcinoma of the bladder (UCB) is a highly prevalent and lethal disease, with an estimated annual global incidence and mortality of 430,000 and 165,000, respectively.1The vast majority of mortality from UCB is due to invasive cancers (invading the muscularis propria and beyond, ≥T2) and advanced disease stages (lymph node or distant metastases, N1-3 and M1). The historic gold standard curative treatment for locally invasive (T2–T3bN0M0) and locally advanced (T2–T4a, N1–3) is radical cystectomy (RC).2Randomized prospective trials have shown that there is a survival benefit when cisplatin-based neoadjuvant chemotherapy (NAC) is administered prior to RC (compared to RC alone).3,4However, the absolute survival benefit with NAC is small in magnitude (6–14% over five years) because only select patients respond to NAC.
Taking into consideration significant toxicities that can result from systemic chemotherapy, an important challenge for oncologists treating UCB is to select patients who will actually benefit from NAC. Here, we will review contemporary patient selection strategies and emerging discoveries that may establish new strategies to select patients for NAC.
The principal behind NAC’s survival benefit is that it may treat micrometastatic cancer lesions prior to clinical detection, thereby complementing RC as a cancer-eradication modality. This notion is supported by subset analysis that demonstrates increased NACassociated survival benefit with higher stage cancers,3though other analyses show no stage-dependent effect of NAC response.5There are no Clinical Laboratory Improvement Amendments (CLIA)-certified predictive tests that can identify patients most likely to derive benefit from NAC, as assessed by either pathologic downstaging (from clinical stage ≥T2 to pathologic stage ≤T1) or survival advantage. Therefore, oncologists must rely on clinical variables that are proxies for high-risk disease. Based on natural history studies of metastasis and cancer specific mortality from independent cohorts, high-risk invasive bladder cancers can be defined by clinical stage T3b–T4a, radiologic evidence of hydroureteronephrosis (a proxy for advanced stage), and/or histologic evidence (via transurethral bladder tumor biopsy) of lymphovascular invasion or variant histology (micropapillary or neuroendocrine features).6A different clinical algorithm suggests that, among patients who receive NAC, patients who are younger (≤60 years) with lower stage disease (T2) have improved response in terms of pathologic downstaging and cancer specific mortality, though direct quantification of NAC benefit was not performed in these studies because NAC and non-NAC cohorts were not compared.7,8
Advances in genomics have offered new insights that may aid patient selection based on specific gene expression profiles. The model system for this comes from instrinsic subtypes of breast cancer identified by Perou et al.,9 which are both prognostic and predictive for specific treatments. We and others have identified intrinsic subtypes of invasive bladder cancer that, similarly, appear to be both prognostic and predictive.10–14For example, basal (The Cancer Genome Atlas [TCGA] Clusters III-IV) bladder cancers demonstrate aggressive clinical behavior,12,13 and a subset of luminal bladder cancers (p53-like, TCGA Cluster II) appear to be resistant to NAC when response is assessed by pathologic downstaging.12,14Furthermore, multiple investigators performing whole genome or targeted sequencing have found an association of mutations in DNA repair genes with sensitivity to NAC (both in pathologic downstaging and survival analyses).15–17Excision repair cross-complementation group 2 (ERCC2), ataxia telangiectasia mutated (ATM), fanconi anemia, complementation group C (FANCC), and retinoblastoma tumor-suppressor (RB1) in particular were identified as correlates of NAC-response. Pathway analyses in lossof- function experimental models may reveal unique mediators that may be leveraged to improve outcomes with NAC.
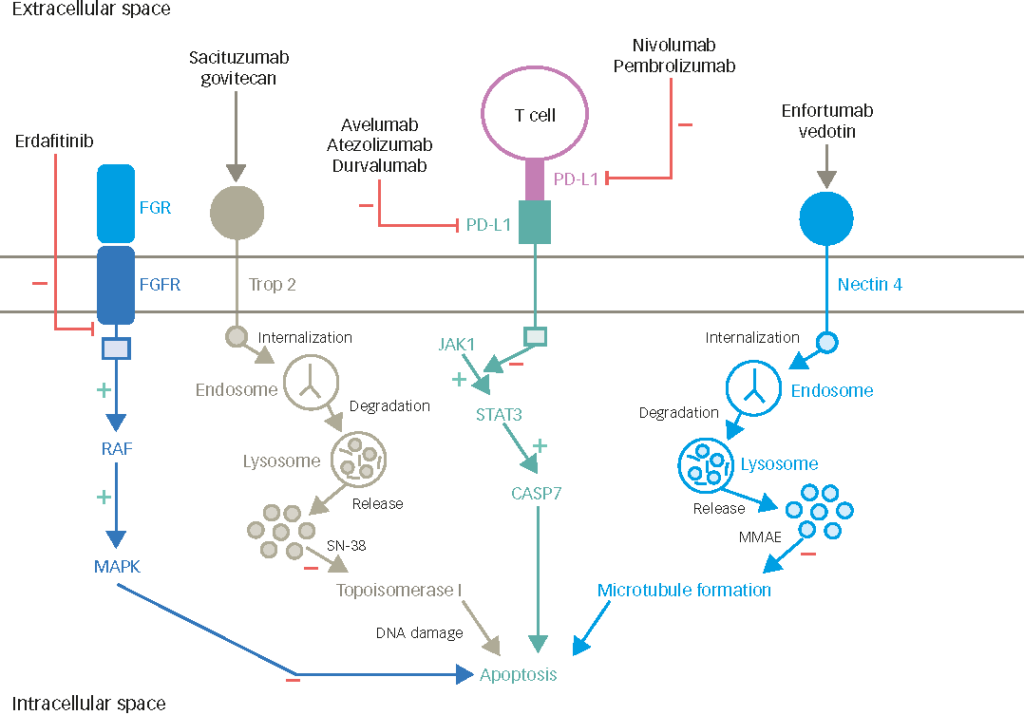
Finally, intrinsic subtypes based on current genomic analyses may also pave the way for patient selection in the newest frontier of systemic treatments: immunotherapy via checkpoint blockade. In particular, a subset of basal bladder cancers (claudin-low, TCGA cluster IV) appear to be highly immune infiltrated, yet express high levels of checkpoint molecules and other immunosuppressive biomarkers, which suggests therapeutic potential for checkpoint blockade in these patients.18Recent data from a phase II trial of atezolizumab (anti-programmed death-ligand 1 [PD-L1]) also suggests that in the p53-like (TCGA Cluster II) subtype, there is a higher relative response to immunotherapy, which may be a viable strategy in this patient cohort that appears to be cisplatin-resistant.19Results of the CO-eXpression ExtrapolatioN (COXEN) trial (NCT02177695) will also be informative for both validation and discovery, as this trial will correlate gene expression profiles and DNA mutations with pathologic responses, survival, and multiple NAC regimens in prospective fashion.20
In summary, the gold standard treatment algorithm for invasive bladder cancer was re-defined by the paradigm of neoadjuvant chemotherapy (NAC). Level I evidence demonstrates a survival benefit with NAC, but precise patient selection will be key to improving outcomes and minimizing toxicity. Recent investigations and emerging data suggest that intrinsic subtypes of invasive bladder cancer based on genomic profiling will form the basis of useful predictive clinical tools to choose systemic chemo- and immune-therapies.