It was estimated that more than 429,000 new cases of urothelial cancer (UC) were diagnosed worldwide in 2012.1 There are limited treatments for locally advanced, unresectable, metastatic, platinum refractory UC.2 As a single agent, doxorubicin has a response rate of 17 % in patients with previously treated and untreated advanced bladder cancer. Complete responses (CRs) are uncommon and the median duration of response was only 3 to 4 months.3–6
Luteinising hormone-releasing hormone receptors (LHRH-Rs) are found on human cells of urothelial origin. Their high expression on the neoplastic cells and minimal expression in non-neoplastic cells, makes the LHRH-R an attractive therapeutic target.7,8
Zoptarelin doxorubicin is an LHRH-cytotoxic hybrid drug, in which an LHRH agonist is coupled to the cytotoxic radical, doxorubicin. The binding permits zoptarelin doxorubicin to accumulate on the surface of cells expressing LHRH-R allowing the uptake of the doxorubicin, thereby exploiting these receptors to gain access to the targeted tumour cells. Once internalised, the cytotoxic properties of doxorubicin provoke the anti-tumour response.9,10
Phase I and II studies of zoptarelin doxorubicin have been reported in females with LHRH-R-positive endometrial and ovarian cancer and have demonstrated that this drug has activity and can be safely given with few side effects.11,12 The first phase I study with zoptarelin doxorubicin included 17 women with epithelial cancers of ovary, endometrium or breast, and which were unresectable or metastatic. Each patient received intravenous doses of 10, 20, 40 or 80 mg/m2 of zoptarelin doxorubicin, six received 160 mg/m2 and seven 267 mg/m2 at 3 week intervals. The half-life of Zoptarelin doxorubicin was estimated to be about 2 hours. Dose-limiting leukopenia and neutropenia were observed only at the highest dose. A total of six patients, three in each of the high-dose groups (three at 160 mg/m2; three at 267 mg/m2), showed responses to zoptarelin doxorubicin. The 267 mg/m2 dose at intervals of 3 weeks was then selected as the starting dose for phase II studies.11 The first phase II trial included 42 patients with taxane-resistant and platinum-resistant LHRH-R positive ovarian cancer. In this study, patients were treated with up to 6 cycles of zoptarelin doxorubicin at doses of 267 mg/m2. Six patients (14.3 %) achieved a partial response (PR) and 16 patients (38.1 %) achieved disease stabilisation with a clinical benefit of 52.4 %. Median time to progression (TTP) was 12 weeks and median overall survival was 53 weeks. The safety profile of the 267 mg/m2 dose was confirmed.12 In the second phase II study, 43 patients with LHRH-R positive, International Federation of Gynecology and Obstetrics (FIGO) III/IV or recurrent endometrial cancer were included and were treated with six courses of zoptarelin doxorubicin at the 267 mg/m2 dose. Two patients had CR (5 %), eight patients had a PR (19 %) and 20 patients had stable disease (47 %). Clinical benefit was thus seen in 70 %. Median TTP was 7 months and overall survival was 15 months.13
In a recent phase I study, zoptarelin doxorubicin was a tolerable agent with activity in men with castration- and taxane-resistant prostate cancer. The dose for further study in this patient population was recommended to be 210 mg/m2 given intravenously every 3 weeks.14
In the US, a zoptarelin doxorubicin phase I clinical trial for metastatic UC was opened in November 2010. (ClinicalTrials.gov identifier: NCT01234519, Study ID Number: AEZS-108-046). Inclusion criteria for the study were:
- Histologically or cytologically confirmed locally advanced or metastatic UC Eastern Cooperative Oncology Group (ECOG) status of 0, 1 or 2 patients with measurable disease and documented progression on at least one prior chemotherapy regimen, which must have incorporated platinum-based therapy.
- Expression of LHRH receptors confirmed by immunohistochemistry on archival cancer tissue.
- Left ventricular ejection fraction (LVEF) >50 %.
Case Report
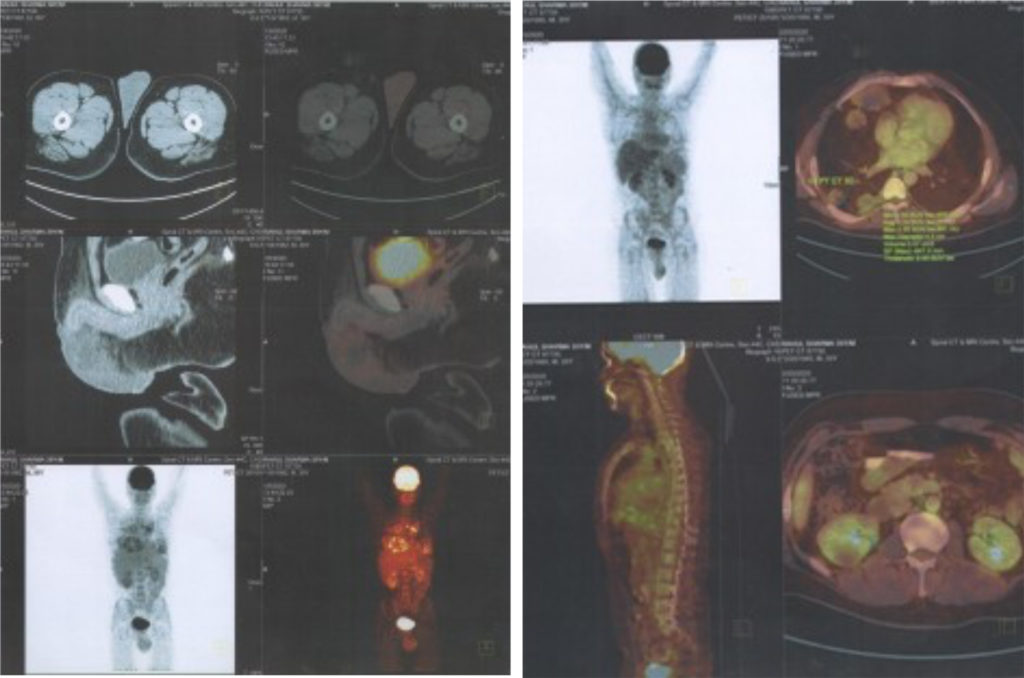
A 66-year-old white male with past medical history of hypertension and emphysema presented with microscopic hematuria in 2007. Several superficial bladder tumours were found and resected. He then had multiple recurrences that required transurethral resection and intravesical treatment. On May 2009, he was found to have high-grade papillary UC of the bladder, and of the prostatic urethra, involving the prostatic stroma. A computed tomography (CT) scan demonstrated multiple enlarged retroperitoneal and pelvic lymph nodes (LNs), the largest measuring 2.1 cm. He underwent four cycles of chemotherapy with cisplatin 70 mg/m2 on day 1 and 1,000 mg/m2 gemcitabine on day 1, 8 and 15, from July 2009 to October 2009 and achieved PR. Following chemotherapy, he underwent radical cystoprostatectomy with lymphadenectomy and neobladder. Pathology showed no residual UC in bladder or prostatic urethra; however, 11 of 24 (46 %) LNs were positive for metastatic disease, including positive nodes in the left obturator space and the left pre-sacral area. The largest metastatic LN measured 1.5 cm. A repeat CT scan in November 2009, 1 month post-operatively, revealed interval development of severe left hydronephrosis and left hydroureter. In December 2009, left nephrostomy tube was placed and a positron emission tomography (PET) scan revealed a left, para-aortic, metabolically active LN. He then received six cycles of carboplatin (area under the curve [AUC]=5) plus paclitaxel 175 mg/m2 between January and April 2010 producing stable retroperitoneal LNs. A CT scan in August 2010 revealed a further increase in size of the retroperitoneal LNs, the biggest now measured 2.5 cm. He then received four cycles of carboplatin (AUC=5), paclitaxel 130 mg/m2 and gemcitabine 750 mg/m2 from September to November 2010. A follow-up CT scan in December 2010 revealed further progression of his disease with additional enlargement of the retroperitoneal lymphadenopathy, the biggest node located in the left para-aortic region now measured 3.5 x 3.7 cm. The patient had entered a clinical trial with a Bcl-2 inhibitor (investigational drug) plus docetaxel 75mg/m2 from January to March 2011.
Unfortunately, a study in March 2011 showed further worsening retroperitoneal LNs and new liver metastasis. (see Figure 1). The patient now had an ECOG performance status of 2, with severe back pain requiring large doses of narcotics. He now developed palpable left cervical LNs. The patient started zoptarelin doxorubicin in a phase I clinical trial (ClinicalTrials.gov identifier: NCT01234519, Study ID Number: AEZS-108- 046), designed for metastatic LHRH-R positive metastatic UC in patients who had failed platinum-based chemotherapy. Patient’s tumour cells were positive (25 %) for LHRH-R by immunohistochemistry. He received 160 mg/ m² of zoptarelin doxorubicin as a 2-hour infusion every 3 weeks for nine cycles from May to October 2011. After two cycles of this drug, all his pain and all palpable cervical LNs disappeared: he stopped all narcotics. He tolerated treatment very well. Toxicities were mostly grade 1–2 with an episode of neutropenic fever (grade 3). A CT scan 2 months after starting treatment and three cycles of investigational drug showed response in all sites of disease (see Figure 2). CR was evident at 20 months posttreatment (see Figure 3). Thirty months (2.5 years) after starting treatment the patient is asymptomatic with no evidence of disease.
Discussion
The standard chemotherapy treatment for advanced disease in patients with UC has been cisplatin-based and with a median survival of 15 months. Unfortunately, long-term survival has been suboptimal – most patients die from their disease.15–17 Once the cancer progresses after firstline cisplatin-based therapy, there are limited good treatment options. The average median overall survival of these patients is 6–9 months.2,3,18–20 A randomised phase III trial with 370 patients compared vinflunine (VFL) plus best supportive care (BSC) with BSC alone as second-line therapy. In the eligible population, the median overall survival was significantly longer for VFL + BSC than BSC (6.9 versus 4.3 months, respectively). As a result of this study, VFL was the first agent to be approved in Europe for second-line treatment in UC.21,22
Doxorubicin, a DNA-intercalating antibiotic, has been used alone, as first-line therapy in advanced UC patients, and in combination with other agents as second-line therapy, with a 17 % response rate.3–6 Zoptarelin doxorubicin, an LHRH-cytotoxic hybrid drug whose rational design covalently couples the carrier D-Lys6-LHRH (an LHRH agonist) to the doxorubicin radical, employs the doxorubicin molecule as its cytotoxic agent. The structure of zoptarelin doxorubicin fully preserves the cytotoxicity of doxorubicin while maintaining the binding affinity of the LHRH carrier.11 As receptors for LHRH are not expressed in high concentrations in most normal tissues, serious targeting related side effects are not expected after treatment with zoptarelin doxorubicin.23–25 LHRH-Rs have been reported in UC7,26 – zoptarelin doxorubicin powerfully inhibited growth of bladder cancer in nude mice.
Zoptarelin doxorubicin exerted a greater effect than doxorubicin alone but with less toxicity.26 Clinical activity and favourable safety have been obtained in patients with LHRH-R positive ovarian, endometrial cancer and prostate cancer.11–14 Ours is the first patient with LHRH-R-positive UC treated with zoptarelin doxorubicin that has been reported.27 Despite failing multiple prior chemotherapies this patient had a CR with a long progression-free survival. Patient is still free of disease at 30 months. The phase I study is completed and closed to accrual28 (ClinicalTrials.gov identifier: NCT01234519, Study ID Number: AEZS-108-046).
Conclusion
Considering the usually poor outcome of progressive UC and the short survival of these patients the effects of zoptarelin doxorubicin in this patient has been remarkable.