X-ray Imaging
X-ray Imaging
The use of X-ray mammography to produce a 2D image of the breast has been used for over 4 decades. Although Albert Solomon first reported the use of X-rays to image mastectomy specimens in 1913,1 it was not until 1939 that Stafford Warren first reported its use in clinical practice.2 Raul Leborgne was the first to report mammography using breast compression in 1949 and through the 1950s and 1960s mammography became increasingly used for breast diagnosis.3 The first commercial dedicated mammography machine was developed in 1966 by the Compagnie Generale de Radiologie4 in France and this machine is the model on which modern mammography is based. Since then there have been incremental improvements in mammographic technology, culminating in the introduction of digital mammography in the 1990s.
Mammography requires very high spatial resolution to detect the features of early breast cancer, particularly microcalcifications. The resolution limitation of digital imaging technology has meant that mammography has been the last X-ray-based radiology technique to transfer from film to digital acquisition, with the required image receptor technology only becoming available since the mid-1990s. Mammography has been used successfully for population screening for breast cancer since the 1960s and mammographic screening is now available in most developed countries. The development of full field digital mammography (FFDM) using high-resolution receptors has provided the opportunity to significantly improve the performance and efficacy of mammography, particularly in screening for breast cancer and the assessment of the mammographically dense breast.5 Breast density is important for two reasons. Firstly women with dense breasts have up to a fourfold increase in breast cancer risk and secondly having dense breasts reduces the chances of mammography detecting breast cancer by up to half. FFDM is equivalent to screen/film mammography in older women5 as breast density is less of a problem, but, over the past decade, clinical trials have shown that here is a significant increase in cancer detection with FFDM in the dense breast. FFDM provides a wider range of grey scale and contrast, translating into significantly better performance in younger women and all women with denser breasts.6 The digital image format also provides the ability to manipulate the image on high-resolution image display screens, allowing for the application of computer-aided detection (CAD) algorithms and the application of multislice sectional imaging (digital breast tomosynthesis [DBT]). DBT is probably the most important recent development in mammography. This technology addresses many of the limitations of 2D imaging where overlapping normal structures result in both false positive and false negative interpretation. DBT obtains up to 20 sectional images of the compressed breast by rotating the X-ray source in an arc over the digital detector using a modified standard mammography unit and then using these images to reconstruct a 3D model of the breast, which can viewed as a stack of slices. Each slice allows better depiction of the structures within that plane and blurring of structures in other planes (see Figure 1). Initial clinical studies have concluded that DBT leads to subjectively clearer delineation and more definitive identification of malignant lesions and their extent.7 Recent trials have shown that DBT greatly improves breast-screening sensitivity, reducing false positive results (FPR) by up to 50 % while at the same time improving breast cancer detection.8 DBT increases the conspicuity of malignant lesions, especially in women with increased parenchymal density, and, equally importantly, reduces the number of women recalled for further investigations that prove to be normal.9
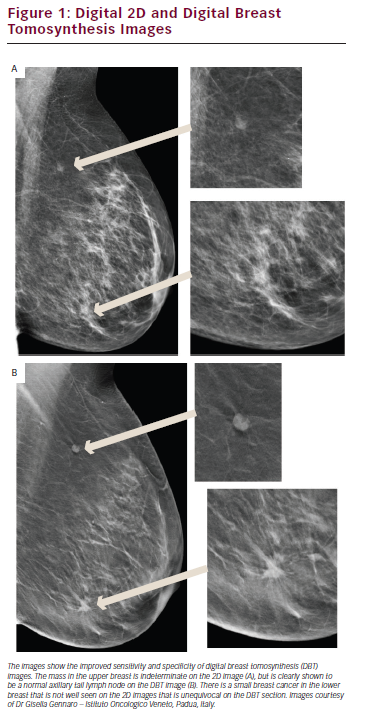
Digital mammography has also provided many logistic advantages that mean that mammography is now much more transferable and archiving much more cost-effective.10 It is likely that DBT will become the standard technique for screening all women with denser breasts and is already being adopted as routine for further assessment of screen-detected abnormalities.
Another new digital mammography method being introduced designed to improve the sensitivity of mammography in the dense breast is dualenergy contrast enhanced digital subtraction mammography. Iodinated contrast is injected intravenously while the compressed breast is imaged on a conventional mammogram machine. Two X-ray exposures each through different metal filters (molybdenum and copper) produce two images that are digitally subtracted to leave an image that shows abnormal focal increased contrast enhancement. Contrast-enhanced mammography (CEM) has the potentially to be a less-expensive alternative to magnetic resonance imaging (MRI) for the assessment of breast tissue vascularity and tissue permeability. Recent clinical trails have indicated that CEM is clinically feasible and enhancement findings are similar to MRI.11 However, larger comparative studies of CEM and MRI are needed in order to further assess this technique and what it might be able to contribute to the existing diagnostic pathways. Dedicated breast computed tomography (BCT) is another X-ray-based technique being developed to overcome the effect of tissue overlap on 2D imaging. The patient lies prone on the dedicated breast CT scanner table with the breasts placed through an opening in the couch. No compression is needed and this makes the patient experience significantly more comfortable.12 BCT uses similar radiation doses to mammography.13 This technology is evolving with the main challenges being achieving the required resolution to detect microcalcifications and design adjustments to ensure adequate imaging of the axillae.14 When these limitations are overcome it is likely that BCT will become an important additional breast-imaging technique. In summary, X-ray mammography, after 50 years, remains the single most important and widely used imaging technique for the investigation of breast disease and for screening for breast cancer. The dramatic improvements in the efficacy of X-ray mammography resulting from the development of digital image acquisition and digital image manipulation mean that X-ray mammography is likely to remain in clinical use for some considerable time.
Ultrasound
Breast ultrasound has long been adopted as the imaging technique of choice for younger women, as an adjunct to mammography for older women, for the further diagnostic evaluation of abnormalities identified on clinical palpation and is the technique of choice for needle breast biopsy. The advantages of ultrasound are that it is widely available, cheaper, more patient friendly and does not involve ionising radiation.15–17
When combined with mammography, ultrasound provides increased sensitivity in detecting cancer compared with mammography alone. The increase in sensitivity for breast cancer has been reported to rise from 74–97 % when mammography is combined with ultrasound instead of clinical examination. Ultrasound can also identify early small (less than 10 mm) node-negative breast cancers that can be cured by excisional surgery at the same time as achieving excellent cosmetic results.18 Ultrasound is particularly effective at increasing good prognosis breast cancer detection in the mammographically dense breast.19 The American College of Radiology Imaging Network (ACRIN) trial demonstrated that combining ultrasound with mammography led to an additional 4.2 cancers detected per 1,000 women with elevated cancer risk due to dense breast parenchyma.20 The majority of these cancers were small and axillary node negative. These findings have prompted some to suggest that supplemental ultrasound should be routine in screening women with dense breasts. However, there are significant drawbacks to the routine use of ultrasound in screening. Firstly, ultrasound is operator dependent, which impacts on its reproducibility. Most studies have used experienced radiologists with specific scanning protocols to perform the examination and it is unlikely that this level of expertise can be provided for the millions of women that with dense breast that would qualify for this additional screening test. Another obstacle in implementing ultrasound as routine in screening is its poor comparative specificity with high FPR, significant increases in benign biopsy rates, and, hence, decrease in the positive predictive value (PPV) of screening for cancer. In screening programmes using mammography alone, the recall rate that results in biopsy is in the range 1–3 % but in the ACRIN trial the recalls that led to biopsy rose to 8.9 % with a resultant decrease in the PPV of 50 %.20
Automated whole breast ultrasound (AWBU) is a new technique designed with the intention of overcoming the problem of operator dependency. A technologist applies the required pressure to ensure the transducer contacts the breast while a computer-controlled mechanical arm moves the transducer in a controlled and reproducible way. The resultant video images and 3D reconstructions are reviewed and read by radiologists. Each scan takes between 10 and 20 minutes to complete following 5–10 minutes of preparation. Initial studies show that AWBU is well tolerated by patients. Recent studies of AWBU in addition to mammography in women with dense breasts at high risk of breast cancer have suggested that AWBU can double the cancer detection rate.21
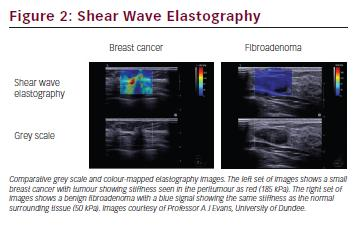
Ultrasound elastography is a complementary ultrasound technique that provides information about the stiffness of tissues. The degree of deformity of tissues is calculated following the passage of an external compression sound wave delivered either manually by the operator (compression elastography) or with the sound itself by measurement of a ‘shear wave’ generated from the ultrasound probe that propagates through tissues (shear wave elastography). Stiffer tissues, such as those infiltrated with cancer, deform less and are stiffer, and can be differentiated from the normal and benign surrounding tissue (see Figure 2).22 Although elastography is not currently used in routine radiological practice for the assessment of breast lesions, its sensitivity and specificity for malignant lesions has been reported to be as high as 97 % and 83 %, respectively.23 Some studies have also reported that increased stiffness of a lesion correlates with prognostic factors such as histological grade and lymph node involvement.24 However, there are still concerns over the variability of operator performance as well as differences in the technical parameters applied to different machines.25
Contrast-enhanced ultrasound (CEUS) is a technique that has been under investigation for some time now in various anatomical contexts including for the imaging of angiogenesis in the breast.26 Microbubble contrast is delivered intravenously and then imaged in realtime as it flows through a breast lesion’s vasculature. Enhancement patterns between malignant and benign lesions have been reported to be significantly different27 and using quantitative kinetic parameters may help with differentiation.28 A more interesting application of CEUS in everyday clinical practice that is subject to current research is in axillary sentinel lymph node identification. This can be performed pre-operatively by injecting microbubble contrast subdermally into the relevant quadrant and then imaging the lymphatic channels with ultrasound.29 If this technique does allow for accurate identification of the sentinel nodes it could prove to be an effective method for early assessment of axillary disease in breast cancer and significantly improve the accuracy pretreatment assessment of the axilla.
Photoacoustic (PA ) imaging is a new and potentially important functional imaging technique that is based on the detection of an ultrasound signal generated on absorption of laser pulses in the near-infrared range. The ultrasound signal varies depending on the oxygenation of tissues because of the differential optical absorption of oxygenated and deoxygenated blood. It is a potential extension to standard diagnostic breast ultrasound as it can image the vasculature and hypoxia within tumours. Following extensive pre-clinical work it is now being assessed in the clinical setting but as yet clear information about its effectiveness and applications are lacking.30
Magnetic Resonance Imaging
Dynamic contrast-enhanced (DCE) breast MRI is currently the most accurate technique available for diagnosing and delineating the extent of invasive breast cancer with many studies consistently reporting sensitivities for breast cancer in all types of breast greater than 95 %.31,32 MRI can identify additional sites of disease in the breast that cannot be seen on mammography or ultrasound and it gives the best correlation with pathological size.33 Following the publication of prospective randomised trials showing probably high levels of effectiveness in high-risk populations, breast MRI is now used widely as the preferred screening method in women with a very high risk of breast cancer (BRCA1/2 mutation carriers) and those who have had previous supradiaphragmatic mantle radiotherapy.34,35
The advantage of DCE-MRI is the combination of morphological features and functional dynamic enhancement characteristics, which give the greatest sensitivity for cancer detection (see Figure 3).36 DCE-MRI is also not affected by breast density. Temporal enhancement characteristics differ between malignant and normal or benign tissues as malignant lesions exhibit much faster contrast enhancement and stronger signal intensity than benign lesions or normal tissue because of an increase in fenestration and functional permeability of blood vessels in neoangiogenic vasculature.38 Enhancement can either show rapid wash-in and wash-out (type III) or a plateau (type II) curve with these two curves seen in 91 % of malignant cases (57 % for type III and 34 % for type II). A gradually enhancing (type I) curve is seen in benign lesions in 83 % of cases.39 The main limitation with DCE-MRI is its variable specificity. Pooled estimates from recent meta-analyses in the diagnosis of breast lesions report specificities of 72 % and 75 %39,40 indicating that while DCE-MRI is excellent for detecting abnormalities in the breast it cannot always confidently determine whether the tissue is malignant, sometimes necessitating a second-look ultrasound and further tissue sampling. Advanced MRI techniques including functional sequences such as diffusion-weighted imaging (DWI) and spectroscopy, as well as highfield scanning have recently been reported to raise the specificity of breast MRI and there is a lot of interest in developing these techniques further for improved clinical evaluation. The increasing availability of 3.0 T scanners, which provide better spatial resolution and faster scanning time compared with the more usual 1.5T, suggest improved overall image quality and confidence in reporting lesions as small as 4 mm.41
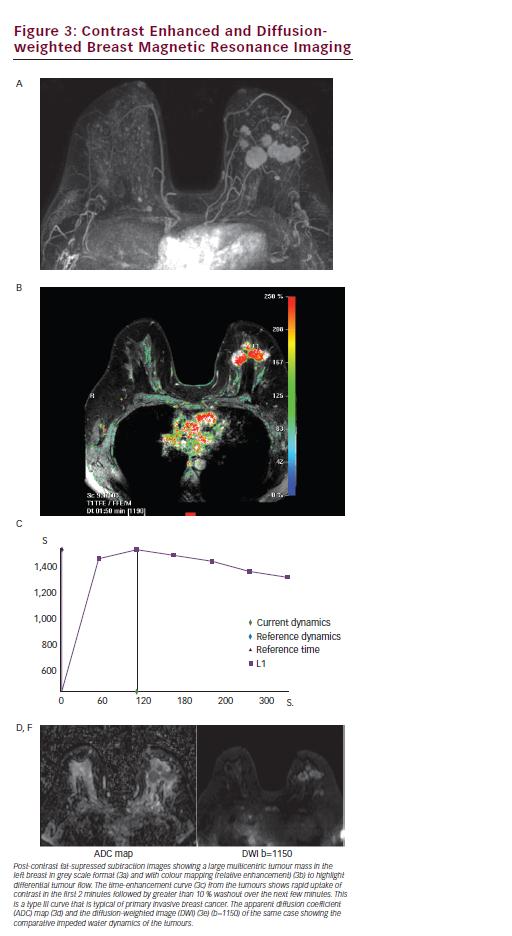
DWI is a functional MRI technique that is reported to improve the specificity of breast MRI from 74 % to 85 %.42 It provides different and complementary information to DCE-MRI, being sensitive to factors that affect the microscopic movement of water molecules over very small distances (<30 μm), such as changes in cellularity, membrane integrity and composition of macromolecules in the extracellular matrix and stroma. Breast cancers demonstrate impeded diffusion because of a high cell density that results in a high signal intensity lesion on DWI and a corresponding low signal focus on the derived apparent diffusion coefficient (ADC) map. Differentiation between malignant and benign breast lesions on DWI is well reported with malignant lesions having low ADC values (0.9–1.61x10-3mm2/s), benign lesions a slightly higher ADC (1.41–2.01×10-3mm2/s),43,44 and normal fibroglandular tissue an even higher ADC (1.51-2.37×10-3mm2/s) (see Figure 3).45 The improved sensitivity as well as specificity afforded by using DWI in conjunction with DCE-MRI could in practice prevent a third of biopsies.46
The ADC value is associated with tumour aggressiveness with highgrade aggressive tumours having a lower ADC than lower-grade tumours.47 The mean ADC values are also lower in patients positive for the proliferation marker Ki67.48 Interestingly the ADC value is reported as higher in women who are oestrogen-receptor negative and herceptin 2 (Her2)-receptor positive with Her2-enriched tumours having the highest median ADC value of all the subtypes.48 The mean ADC value of triple-negative breast cancers is also significantly higher than for other subtypes,49 likely as a result of central intratumoral necrosis resulting in increased free diffusion of water.50
The prognostic value of the ADC remains questionable. The majority of reported studies have found no correlation of ADC with traditional prognostic factors such as lymph node status, tumour size and Her2 status.48,51,52 One paper, however, does report that lower ADC values correlate with lymph node status, tumour grade and tumour size.53 These discrepancies are likely related to the numbers employed in each study and in part to the way regions of interest were drawn and further research in this area is warranted. Overall, however, the ADC is considered a promising adjunctive imaging biomarker that can identify highly aggressive breast cancer.
Proton magnetic resonance spectroscopy (1H-MRS) is another functional technique that provides information in vivo on the molecular content of tissues and their metabolism and can also improve the specificity of DCE-MRI up to 88 %.54,55 In breast cancer, high levels of cholinecontaining metabolites are involved in phosopholipid metabolism and indicate cellular membranes in proliferation resulting in a triplet peak at 3.22 ppm of free choline, phosphocholine and glycerophosphocholine. Choline is almost undetectable in normal breast tissue and a peak at 3.25 ppm can indicate malign tissue;56 however, the presence of choline does not confirm the presence of malignancy. There is promising recent data on the value of MR metabolic profiling in predicting long-term survival and monitoring of treatment response following neoadjuvant chemotherapy with higher total choline levels and lower lactate levels observed in patients surviving more than 5 years. Long-term survival was also best predicted by a fall in glycerophosphocholine post treatment, which may provide valuable information for individual prognosis if spectroscopy could be integrated into clinical assessment.57
Nuclear Medicine Techniques
Until now, nuclear imaging of the breasts has been of limited value but new developments mean that it could have a future role in the evaluation of suspected breast cancer. The investigations available include all common forms of radionuclide imaging including single photon emission CT (SPECT) and positron emission tomography (PET), as well as breastspecific techniques, such as scintimammography and positron emission mammography (PEM). The advantage of all these methods is that they provide functional information and therefore help determine the nature of a lesion. Nuclear medicine (NM) can be particularly useful in the investigation of palpable lesions in women with dense breasts that cannot be identified on mammography with reported sensitivity and specificity of 87.8 % and 87.5 %, respectively.58 However, for nonpalpable, subcentrimetre lesions the performance falls significantly, with sensitivity of less than 50 %.59 This is because, with all NM techniques, spatial resolution is poor. Other ways to combat this disadvantage is the combination of SPECT with dedicated breast CT to offer superior spatial resolution. Also, new, more specific, breast cancer tracers are being developed such as oestrogen and Her2-labelled radionuclides. Overall, these methods may be helpful as an adjunct to established practice to identify malignant lesions but cannot replace the need for histological sampling especially for small lesions.60 Further applications may include staging and evaluation of suspected recurrence if mammography and MRI are equivocal but more research and advances are needed before NM techniques can be advocated for routine use in the detection and further evaluation of primary breast cancer.
Dual-modality Imaging
Although a combination of modalities can be used in the investigation of breast cancer, dual-modality systems have been prototyped with the aim of performing two imaging modalities using the same equipment. This has the potential to improve speed and more accurate coregistration of abnormalities. Automated ultrasound can be combined with CT or breast MRI and this should make biopsy easier to achieve. Other examples are the combination of DBT and scintimammography and 18FDG-PET-MRI where anatomical and functional techniques together have potential to provide a more accurate assessment.61
Experimental Techniques
New research has investigated how breast cancer can be detected, away from traditional radiological methods. As passage of X-rays is easier through normal breast adipose tissue than malignant lesions so do other wave forms, such as light and heat. The absorption of light and heat can be measured by PA and thermoacoustic techniques, respectively, by measuring the sound created by the different wave forms. Due to the difference in the dielectric properties of malignant lesions compared with normal breast tissue this technique can detect cancer and in vivo results have shown promise.62 Similarly, functional information can be obtained using other wave forms such as microwave and infrared light. Measurements such as haemoglobin, oxygen saturation and water content can be obtained non-invasively and displayed as an image. Abnormal angiogenesis can affect these parameters in malignant lesions and therefore they can be differentiated from healthy tissue.63 The above techniques are experimental stages and far from being clinically applicable.
Conclusion
Imaging of the breast is now regarded as essential for detection and diagnosis of breast disease. Mammography and ultrasound remain the standard front-line techniques and dramatic improvements in digital technology mean that these techniques remain highly effective for both screening and symptomatic disease evaluation. In the screening setting it is likely that DBT will soon become routine, with resultant improved detection and dramatic decrease in FPRs. Contrastenhanced MRI is now established as the most sensitive technique for the breast and new technologies in MRI hardware, imaging sequences and breast-specific contrast agents will mean increasing use of breast MRI in routine practice for detection, assessment of disease burden, and response to treatment. There are a number of novel technologies and reiterations of older technology being evaluated for the breast, but these have yet to be proved in clinical practice.