It has been clearly established that non-small cell lung cancers (NSCLCs) associated with known molecular drivers (e.g., rearrangement of the ALK gene, or mutations activating the epidermal growth factor receptor [EGFR] gene) respond favorably to targeted, first-line therapies compared with standard chemotherapy.1,2 The National Comprehensive Cancer Network recommends EGFR and ALK testing (category 1), as well as ROS1, BRAF and programmed death-ligand 1 (PD-L1) testing (category 2A), for patients with metastatic non-squamous NSCLC.3 However, numerous practical constraints prevent the identification of genetic alterations that can be treated with targeted therapies, especially for patients with advanced NSCLC. Initial diagnostic biopsy procedures, which are often minimally invasive (e.g., fine needle aspirations), frequently yield insufficient tissue for molecular testing.4 Performing a second, more invasive biopsy can address this situation, but these procedures are associated with significant risk and cost.4 Consequently, patients are often unable or unwilling to tolerate these secondary procedures. Additionally, because of intra-tumor heterogeneity and potential differences between primary and metastatic tumors, biopsied tissues often do not contain the full complement of genetic mutations that characterize a patient’s disease.5–7
Recent advances in liquid biopsy methodologies now make it possible to molecularly characterize tumors from a peripheral blood draw to gain information that reflects systemic representation of a patient’s existing tumor clones.5–9 Biocept, Inc. (San Diego, CA, US) has developed liquid biopsy technologies to detect tumor-associated alterations in: 1) circulating tumor cells (CTCs)10–12 shed from primary or metastatic tumors, and 2) circulating tumor DNA (ctDNA)13,14 derived from apoptotic or necrotic tumor cells. Previous analyses involving several tumor types (including NSCLC) have shown high concordance between molecular results obtained directly from biopsied tissue and results obtained via Biocept’s liquid biopsy.13,15,16 This case report demonstrates the utility of liquid biopsy to complement tissue molecular testing. A patient with NSCLC was subjected to multiple biopsy procedures, which each failed to identify a molecular driver. Before initiating systemic chemotherapy, a blood draw was sent to Biocept, and a rearrangement of the ALK gene was detected. Administering a series of ALK inhibitors to the patient resulted in an outstanding clinical response and extended the patient’s life, maintaining reasonable quality of life.
Materials and methods
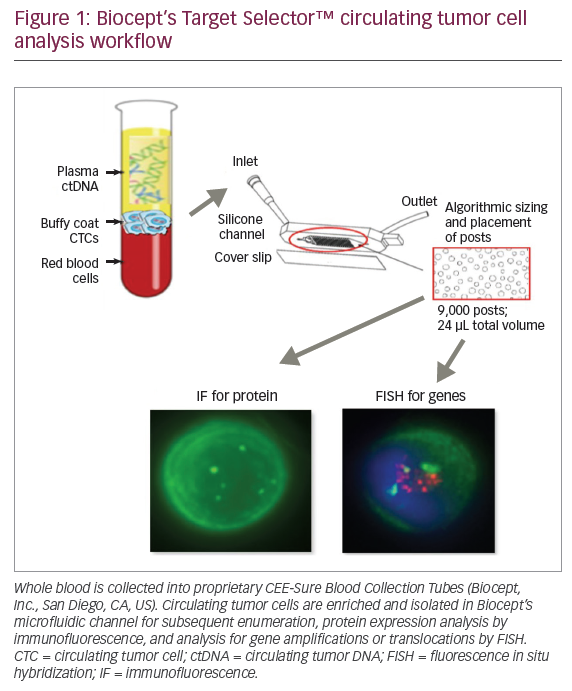
Peripheral blood was collected into 10 mL Biocept CEE-SureTM Blood Collection Tubes (Biocept, Inc. San Diego, CA, US), validated for CTC preservation and analysis when maintained at ambient temperature up to 96 hours. Samples were shipped to the company’s Clinical Laboratory Improvement Amendments (CLIA)-certified, College of American Pathologists (CAP)-accredited laboratory for processing. CTC enrichment in the company’s patented microchannel was performed as previously described.10,11 Figure 1 depicts Biocept’s CTC analysis workflow. ALK gene translocations were detected by fluorescence in situ hybridization (FISH) on the Biocept CTC platform. Analytical validation of the company’s ALK assay was performed with an ALK-positive cell line. Clinical validation utilized blood samples collected from patients with known ALK tissue status. Biocept’s ALK assay is capable of detecting a single ALK-rearranged CTC with clinical sensitivity of 91%.
Case presentation
In April 2014, an 85-year-old white female reported night sweats during an office visit. She had a history of stage IV-B follicular B-cell non-Hodgkin lymphoma, which was in remission following single-agent rituximab treatment in 2010. She was a never-smoker. Imaging studies were requested, and an initial positron emission tomography-computed tomography (PET-CT) scan revealed increased fluorodeoxyglucose (FDG) uptake in a left upper lobe nodule with both mediastinal and celiac axis adenopathy (Figure 2A). Magnetic resonance imaging (MRI) of the brain was normal. Due to the atypical presentation for recurrent lymphoma and the possibility of a second primary tumor, a biopsy was offered. The patient refused a traditional lung biopsy, so an endoscopic ultrasound-guided fine needle aspiration was used to biopsy the celiac axis node. This procedure was non-diagnostic. Thus, she was treated empirically with rituximab for four cycles.
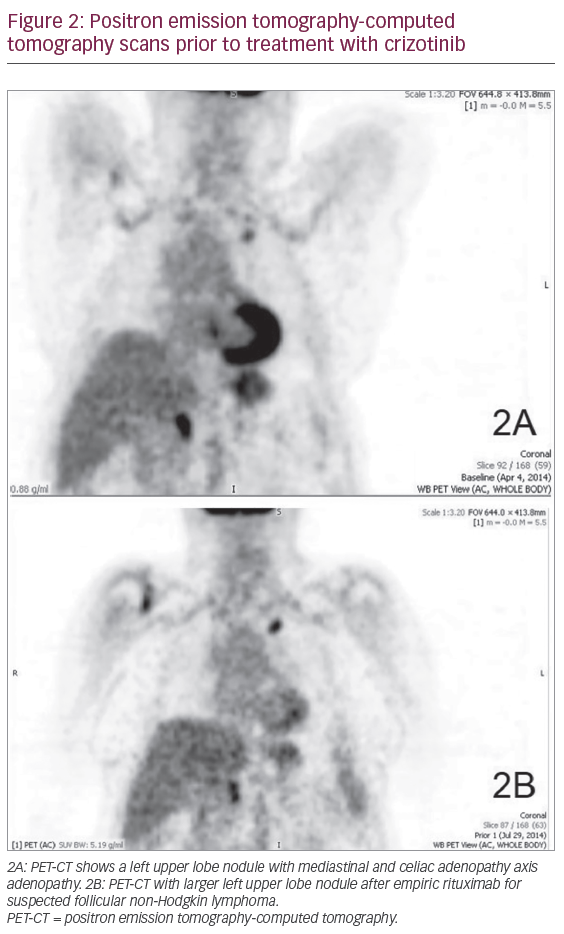
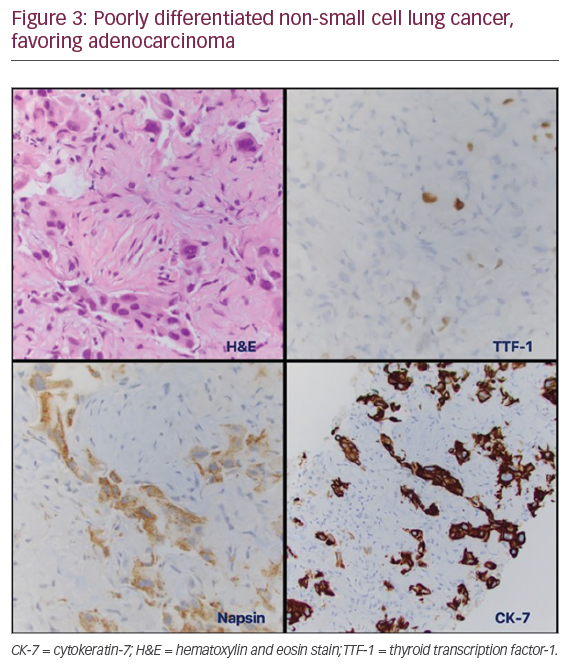
In July 2014, a PET-CT scan following rituximab treatment revealed worsening findings. This included a 30% increase in size of the left upper lobe nodule with a standardized uptake value of 6.8, now with new hilar and higher mediastinal uptake (Figure 2B). Based on this result, the patient agreed to undergo additional diagnostic studies. A fiberoptic bronchoscopy with endobronchial ultrasound biopsy of the mediastinum was performed in September 2014, but the procedure was non-diagnostic. The patient was then subjected to a minimally-invasive navigational bronchoscopy procedure with biopsy of her left upper lobe lesion. This procedure was also non-diagnostic. Finally, in October 2014 a computed tomography (CT)-guided percutaneous biopsy of the left lung nodule was performed (Figure 3). The pathology report concerning the biopsied tissue indicated poorly differentiated NSCLC, favoring adenocarcinoma. The tumor was cytokeratin-7, Napsin, and thyroid transcription factor-1 positive. Molecular studies came back negative for ALK translocation via FISH. However, there was insufficient tissue to test for the presence of EGFR mutations. When the patient was asked to endure another biopsy procedure to test for EGFR abnormalities, she refused. Treatment options for advanced NSCLC with unknown EGFR/ALK status include systemic chemotherapy with platinum doublet, or palliative care.
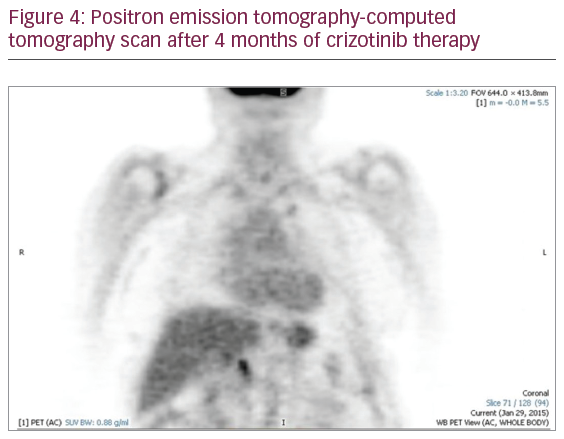
In an attempt to complete her tumor molecular profiling and ensure that the appropriate course of action was being taken to treat this patient, a blood sample was collected in November 2014 and sent to Biocept laboratories for blood-based testing (liquid biopsy). Biocept’s liquid biopsy test detected an ALK-rearrangement in CTCs by FISH. Abnormalities in EGFR were not detected. Based on this molecular information, the patient was prescribed crizotinib (a MET inhibitor that also inhibits ALK and ROS1) in December 2014. Following 8 weeks of crizotinib treatment, a PET-CT scan was performed to assess disease status. This imaging analysis revealed a dramatically reduced tumor load (i.e., the tumor cells had responded to crizotinib treatment). There was an almost complete interval reduction in radiotracer uptake and size of her left upper lobe nodule, mediastinal, hilar, and upper abdominal adenopathy (Figure 4).
The patient remained asymptomatic on therapy with crizotinib for 13 months (until January 2016). At this time, she began to show evidence of dyspnea associated with left-sided pleural effusion. A thoracentesis was performed in late February 2016, showing adenocarcinoma. Thus, in March 2016 she was started on second-line therapy with the ALK inhibitor ceritinib, but unfortunately developed excessive gastrointestinal toxicity. Treatment with ceritinib was stopped after only 2 weeks. Once the patient’s toxicity resolved, she was started on the ALK inhibitor alectinib (600 mg orally, twice daily) with excellent tolerance. By this point, she had had a PleurX™ catheter (BD, Reading, UK) placed for palliative management of her malignant pleural effusion. Within 4 weeks of treatment with alectinib, her effusion resolved completely and the PleurX catheter was discontinued. Following this the patient had minimal symptoms.
In July 2016 she presented to the emergency room with angioedema secondary to an angiotensin-converting-enzyme (ACE) inhibitor. She required emergent tracheotomy with endotracheal intubation, resulting in a prolonged stay in the intensive care unit with severe deconditioning. The patient decided not to pursue additional therapy for malignancy at that point and was discharged with hospice care. She passed away peacefully in late October 2016.
Discussion
Here we demonstrate liquid biopsy as a sensitive and viable option to obtain molecular profiling information where clinical challenges exist with current, conventional methods. This case study exemplifies such situations; the patient refused to undergo a biopsy in one instance, and tumor tissue amounts were inadequate for molecular testing when biopsies were performed. Supplementing traditional tissue-based methods with liquid biopsy was key for identifying an ALK gene rearrangement in this patient who was subsequently prescribed treatment with an ALK inhibitor.
Recent literature has addressed the potential benefits and difficulties of burgeoning ctDNA and CTC methodologies, along with liquid biopsy’s capabilities for disease management of patients with various cancers, including NSCLC.5–9,17,18 Further studies are required to demonstrate the clinical validity and utility of liquid biopsy for widespread clinical practice applied to different biomarkers across various cancer types.17 Nevertheless, the use of plasma ctDNA in specified clinical settings to identify EGFR mutations for guiding targeted tyrosine kinase inhibitor (TKI) treatment decisions in lung cancer is supported by recommendations 16 and 17 of the recently updated molecular testing guidelines from the CAP, the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP).18 As the US Food & Drug Administration (FDA) has approved the cobas® EGFR Mutation Test v2 (Roche Molecular Systems, Inc., Branchburg, NJ, US) for use with plasma ctDNA,19 one can anticipate approval of additional liquid biopsy-based tests in the future.
Detection of ALK rearrangements in patients with NSCLC has been demonstrated in blood plasma, platelets, and CTCs.20–24 For example, Nilsson et al. analyzed 77 patients with NSCLC, using reverse transcription polymerase chain reaction to identify EML4-ALK gene fusions in the plasma or platelets of 38 individuals.21 The study reported a correlation of ALK rearrangement detected in platelets with progression-free survival (PFS) and overall survival in a group of patients treated with crizotinib. Patients in whom ALK was negative on treatment had a PFS of 16 months compared to 3.7 months in patients with persistent ALK rearrangement detection. The presence of an ALK rearrangement in blood was also monitored in one patient during the course of crizotinib treatment. In serial blood collections of this patient, the detection of EML4-ALK reflected therapeutic response and subsequent disease progression. EML4-ALK was detected at baseline prior to therapy and was undetectable after crizotinib treatment was initiated. EML4-ALK was again detected when therapy stopped for one month due to appendicitis. Upon resuming treatment, EML4-ALK was not detected. Finally, the ALK fusion was detected 2 months before disease progression was determined radiographically.21 The study highlights the potential of applying liquid biopsy to monitor drug response and the early detection of resistance, thus providing a valuable supplement to traditional methods for tracking and managing patient disease.
Liquid biopsy CTC methodologies have evolved considerably in recent years. In addition to detection and enumeration, technological developments have enabled characterization of individual CTCs to include analysis of expressed protein, RNA, and DNA. Recent reviews provide an overview of numerous CTC isolation methods and potential clinical applications.6,7,9,25
CTCs are amenable to FISH analyses for detecting gene amplifications and translocations. Evaluation of gene translocations using ctDNA have the technical challenge of requiring prior knowledge of chromosomal breakpoints associated with large genetic rearrangements, often accomplished by whole-genome sequencing. Gene fusions with rarer, unknown breakpoints may remain undetected by these ctDNA techniques. The described ctDNA limitations for detecting gene rearrangements are circumvented by Biocept’s FISH analyses of CTCs; hybridization of nucleic acid probes to complementary chromosomal DNA requires no specific sequence knowledge of gene fusion partners.
In summary, this case illustrates the utility of Biocept’s blood-based liquid biopsy. Identification of the ALK translocation in CTCs led to a sequential targeted therapy, which included crizotinib followed by alectinib, resulting in an outstanding clinical response. The patient enjoyed 18 months of improved quality of life following the initiation of treatment. Posterior complications after initiation of therapy were unrelated to cancer. Importantly, initial analysis of the tissue biopsy failed to detect the ALK rearrangement by FISH. It is unclear what led to this discordance, but tumor heterogeneity is the most likely explanation (i.e., the portion of the tumor retrieved via CT-guided biopsy did not contain this genetic lesion). Thus, liquid biopsy provided a more complete and accurate assessment of the genetic alterations driving this tumor, ensuring that the patient received the most appropriate and beneficial course of treatment.