Cancer treatment has expanded rapidly in recent years as advancements in the fields of tumour biology and molecular diagnostics have informed the development of targeted therapies, improving survival in patients with oncogene-addicted cancers with therapeutically relevant molecular lesions. Osimertinib has become the standard-of-care treatment in the USA and elsewhere for classical epidermal growth factor receptor (EGFR) driver mutations such as exon 19 deletions and exon 21 L858R substitutions that are sensitive to tyrosine kinase inhibitors (TKIs), in both the metastatic and the adjuvant settings. Nevertheless, the median duration of response (DOR) in the first line is about 17 months,1 and there are many additional EGFR mutations that do not respond to standard TKIs due to an inability of the molecule to bind the mutant receptor; the most common is the EGFR exon 20 insertion (ex20ins).
Amivantamab is a novel anti-EGFR/c-MET bispecific antibody that received US Food and Drug Administration (FDA) Breakthrough Therapy designation in March 2020 and subsequently accelerated approval in May 2021 following promising early data from the CHRYSALIS phase I study, which demonstrated both safety in advanced pre-treated non-small cell lung cancer (NSCLC) as well as clinical activity in NSCLC with EGFR ex20ins and classical EGFR mutations resistant to third-generation TKIs.2–4
This review outlines the antibody design, mechanism of action, pharmacological profile, relevant preclinical and clinical studies, and safety and tolerability of amivantamab, a promising therapeutic for non-classical EGFR ex20ins mutated and TKI-resistant NSCLC.
Targeting EGFR driver mutations in non-small cell lung cancer and clinical needs
EGFR, a transmembrane receptor tyrosine kinase in the ErbB family, mediates multiple downstream signal transduction pathways via ERK/MAPK3, PI3K/AKT and STAT that influence proliferation, apoptosis and cellular migration.5,6 EGFR mutations are common drivers of NSCLC, with the frequency of EGFR mutations varying by ethnicity, representing approximately 15% in Caucasian populations and 50% in Asian populations,7 particularly in light/never smokers.
Clinically relevant activating mutations in the EGFR gene occur in exons 18-21 encoding the tyrosine kinase domain, and this heterogenous group of alterations, including insertions, deletions and point mutations, has variable prevalence, prognosis and response to targeted therapies. Classical EGFR primary driver mutations, such as exon 19 deletions and exon 21 L858R point mutations, account for approximately 85% of EGFR mutations in NSCLC and are exquisitely sensitive to anti-EGFR TKIs. However, resistance to first- and second-generation EGFR TKIs (gefitinib, erlotinib, afatinib and dacomitinib) develops inevitably through secondary EGFR mutations, such as exon 20 T790M mutations or upregulation of other tumour growth and metastasis-promoting pathways, such as c-MET.8,9 The third-generation EGFR TKI osimertinib was developed to target tumours that acquired a T790M resistance mutation.1 With outstanding responses, osimertinib is currently approved as both a first-line therapy and as a salvage therapy for patients who progress on first- and second-generation TKI treatment. Yet, despite this success, resistance to osimertinib still develops through other on-target resistance mutations, such as EGFR C797S or other EGFR mutations or through MET amplification as a bypass pathway, as well as other non-EGFR/MET pathways or by unknown or unidentified mechanisms,10 with a median DOR of about 12–17 months.1,11 A clinical need for advancements in the treatment of classical EGFR-mutated NSCLC remains.
Insertions in exon 20 of the EGFR gene are the third most common subgroup of EGFR mutations, representing 9–12% of EGFR mutations in NSCLC and 2–3% of all lung adenocarcinomas.12,13 A heterogenous group of mutations, EGFR ex20ins are typically frame insertions or duplications between amino acids 762 and 774 of EGFR, encoding the C-helix and subsequent loop within the tyrosine kinase domain.5,12,14 Unlike classical EGFR mutations, ex20ins do not alter the adenosine 5’-triphosphate (ATP)-binding capacity of EGFR nor do they enhance affinity for ATP-competitive TKIs.13,15 Rather, C-helix reorientation causes steric hindrance that renders current TKI therapies ineffective, as evidenced by response rates to erlotinib, gefitinib, afatinib and osimertinib of 0–8%,16–20 leaving chemotherapy as the standard first-line approach.21–23 As these are oncogene-addicted tumours, therapies that successfully target the ex20ins would be expected to improve outcomes. Currently, the only FDA-approved targeted treatment for tumours with EGFR ex20ins mutations is amivantamab following progression on chemotherapy.
Identification of EGFR mutations by next-generation sequencing of tumour tissue or circulating tumour DNA (ctDNA) in plasma is used to determine eligibility for and choice of anti-EGFR targeted therapy. Along with the accelerated approval of amivantamab for EGFR ex20ins, the FDA approved Guardant360® CDx (Guardant Health, Inc., Redwood City, CA, USA) as a companion diagnostic. Guardant360 CDx is a liquid biopsy test using next-generation sequencing of ctDNA to detect alterations in common driver genes, including EGFR ex20ins. Among patients in the CHRYSALIS trial, EGFR ex20ins was initially identified by next-generation sequencing of tumour tissue in 94% and by plasma in 6%. Retrospectively, central testing using the Guardant360 CDx tool identified an EGFR ex20ins in 79% of available patient plasma specimens.24,25
Amivantamab as a novel EGFR and MET inhibitor
Amivantamab (JNJ-61186372) is a fully human bispecific immunoglobulin G1 (IgG1) antibody targeting both EGFR and c-MET.2 The c-MET oncoprotein is overexpressed in many NSCLC tumours at baseline, including EGFR-mutated tumours, and c-MET amplification occurs in 5–22% of tumours with acquired resistance following EGFR TKI treatment.26–29 Amivantamab was designed to both delay resistance in TKI-naive tumours and combat resistance in TKI-resistant tumours. Initial in vitro studies found that amivantamab was able to bind to both EGFR and c-MET simultaneously with high affinity, and sequestered the two proteins in a heterodimer, preventing homodimerization and downstream signalling.30,31 Amivantamab was also designed to have low core fucosylation,28 a modification that increases binding to Fcγ-receptors and enhances antitumour immune cell functions.32
Preclinical studies have highlighted the multiple mechanisms by which amivantamab induces antitumour effects (Figure 1). Amivantamab induced natural killer-cell-mediated, antibody-dependent cellular cytotoxicity in multiple cell lines bearing classical EGFR mutations and c-MET amplifications, and also EGFR ex20ins and T790M mutations.28,31,33 In addition, amivantamab mediated tumour-cell interaction with macrophages and monocytes, resulting in trogocytosis of EGFR and c-MET receptors from the tumour cell surfaces to the immune cell surfaces and thus downmodulation of signal transduction and downstream pathways.33 Finally, additional preclinical data suggest Fc-independent mechanisms of action are triggered by inhibition of ligand binding and internalization of EGFR and c-MET receptors, leading to decreased receptor expression and further blunting of tumourigenic growth signals.2,31
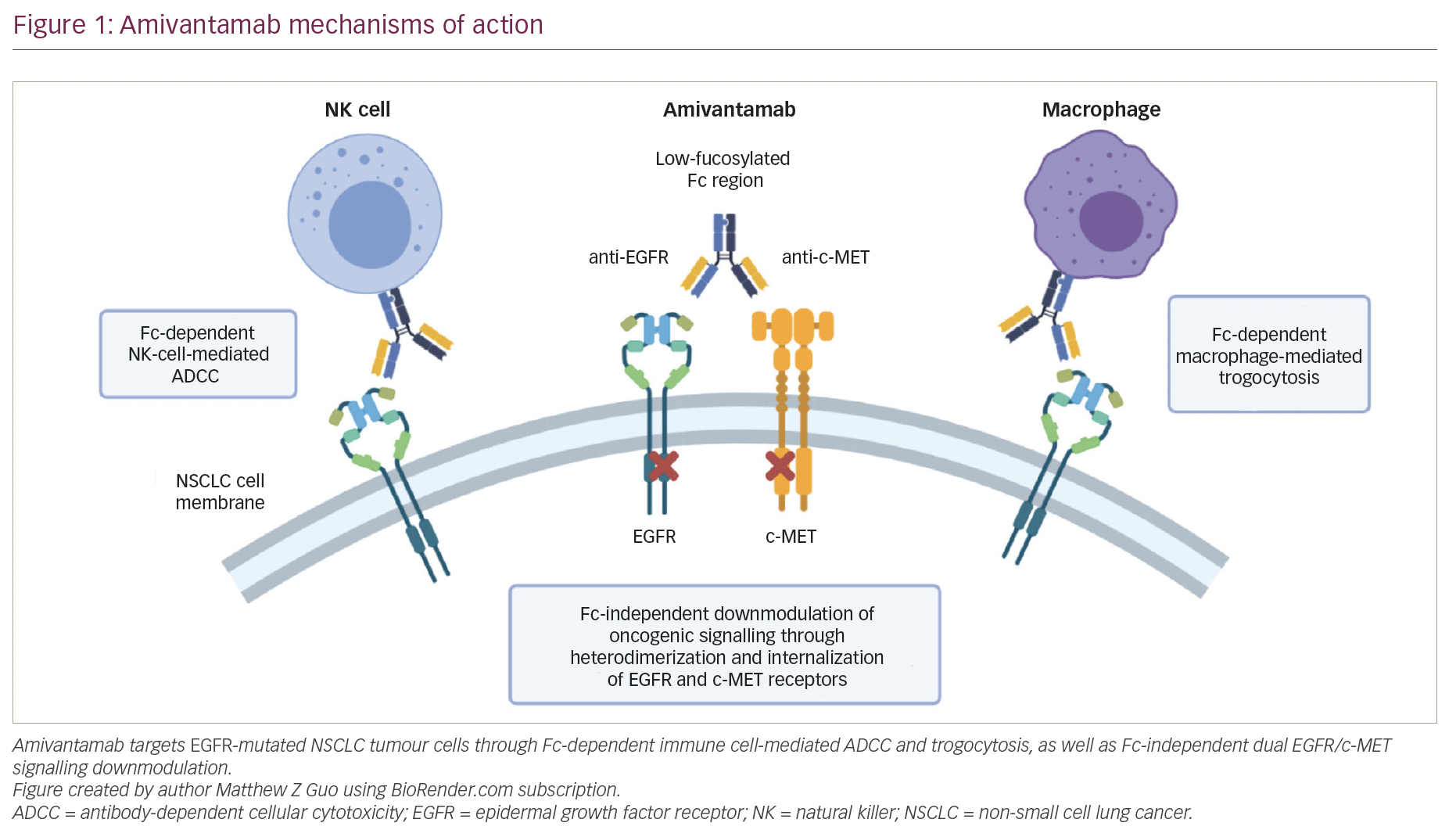
Overall, preclinical data suggested that amivantamab is effective in treating tumours with classical EGFR mutations, as well as ex20ins mutations and common TKI-resistance mutations. Clinically, by binding cell surface receptors instead of their intracellular tyrosine kinase domains, amivantamab is expected to overcome resistance and function mechanistically differently from small-molecule TKIs. Nevertheless, other monoclonal anti-EGFR antibodies, such as cetuximab, have met with limited efficacy in NSCLC and have substantial toxicity.34 The dual-targeting mechanism of amivantamab is hypothesized to increase avidity to tumour cells while decreasing off-target effects, overcoming some of the limitations of monospecific anti-EGFR antibodies like cetuximab.
Clinical data for amivantamab in EGFR exon 20-mutated non-small cell lung cancer
Clinical efficacy of amivantamab in advanced NSCLC has been assessed in the ongoing multi-cohort phase I CHRYSALIS study. The trial, beginning in May 2016, enrolled 460 participants with metastatic or unresectable EGFR-mutated or wild-type NSCLC who had progressed following either EGFR TKI therapy or platinum-based chemotherapy.35
The CHRYSALIS study dose escalation (part 1) determined the amivantamab recommended phase II dose of 1,050 mg for patients weighing <80 kg and 1,400 mg for patients ≥80 kg. The dose-expansion (part 2) study includes various prespecified cohorts segregated by EGFR and c-MET mutation profile.35 Initial data for patients from the ex20ins cohort included 39 response-evaluable patients, 29 (74%) of whom had previously received platinum-based chemotherapy, 6 (15%) were treatment-naive and 4 (10%) had received other therapies. The overall response rate (ORR) was 36% with median DOR of 10 months. The total clinical benefit rate was 67% with median progression-free survival (PFS) of 8.3 months. The cohort included 13 distinct ex20ins mutations, and activity was observed for all mutations.4 Such responses are considerably more favourable than current outcomes of median PFS of 4.1 months for EGFR ex20ins NSCLC treated with standard-of-care chemotherapy.21,22
Follow-up data in 2021 expanded on the post-platinum EGFR ex20ins cohort, reporting outcomes for 81 patients with ORR of 40% and a total clinical benefit rate of 74%. Median DOR was 11.1 months, median PFS was 8.3 months and median overall survival was 22.8 months.25 These findings prompted amivantamab FDA-accelerated approval to address the unmet need for patients with chemotherapy-refractory NSCLC with EGFR ex20ins mutations.
Amivantamab in TKI-resistant EGFR-mutated non-small cell lung cancer
In classical EGFR exon 19-deleted and L858R-mutated tumours, third-generation TKIs, like osimertinib, are standard first-line treatment, yet there are no approved targeted therapies following progression without an alternative actionable mutation. Resistance to osimertinib develops through diverse mechanisms, including increased MET expression, providing a bypass pathway for dysregulated growth and proliferation signalling.8,9 Amivantamab is expected to have antitumour effects in these cases by dual targeting of EGFR and MET, and preliminary findings of the CHRYSALIS study showed that a small number of patients with c-MET-amplified tumours following a third-generation TKI responded to amivantamab.3
Osimertinib resistance also commonly develops through a C797S mutation that prevents osimertinib binding at the tyrosine kinase domain.10 Because the C797 residue is intracellular, amivantamab is hypothesized to have activity against this mutation,31 and some patients in the CHRYSALIS trial who developed C797S mutations following third-generation TKI therapy responded to amivantamab.3 In addition, by binding on the cell surface, amivantamab may overcome other resistance mechanisms in addition to c-MET amplification and C797S mutation that constitute a majority of post-osimertinib progression.
The combination of amivantamab with the novel third-generation anti-EGFR TKI lazertinib is also under investigation in osimertinib-resistant tumours in a dose-expansion arm of CHRYSALIS. Initial data from 45 patients revealed a 36% confirmed response rate with median DOR not reached at median follow-up of 8.2 months. Additional biomarker analysis revealed increased rates of response in tumours with osimertinib-resistance mutations or amplifications in EGFR/MET (response rate 47%) compared with non-EGFR/MET mechanisms of resistance (response rate 0%), and in tumours with high EGFR/MET protein expression (response rate 90%) compared with low expression (response rate 10%).36
Clinical administration of amivantamab and management of toxicities
Amivantamab 1,050 mg for patients <80 kg and 1,400 mg for patients ≥80 kg is administered intravenously with loading doses once weekly for the first 28-day cycle and then every 2 weeks for following cycles. To monitor for and reduce infusion-related reactions, the first dose should be divided into a 350 mg flat dose on day 1 with the remainder of the dose on day 2, while subsequent doses in cycle 1 and beyond can be given as a single full-dose infusion.
Adverse events (AEs) among all patients on the CHRYSALIS study treated with amivantamab at the recommended phase II dose (n=258) most commonly included rash (78% any grade, 3% grade ≥3), infusion-related reaction (65% any grade, 2% grade ≥3) and paronychia (40%), followed by stomatitis (19%), pruritis (19%) and diarrhoea (11%). Thirty-nine percent of patients had grade ≥3 AEs, and 16% were considered treatment-related grade ≥3 AEs. Treatment-related AEs uncommonly led to dose reduction (10%) and discontinuation (3%). There were no reports of treatment-related death.25
Infusion-related reactions should trigger immediate infusion cessation and patient evaluation with acute management of respiratory symptoms, hypotension or anaphylaxis. In stable patients following symptom resolution, the infusion can be restarted at 50% reduced rate and titrated to tolerance. In study populations, nearly all infusion reactions occurred with the first infusion and rarely impacted subsequent treatments, similarly to exposure to other monoclonal antibodies like daratumumab.25,37 Patients can be premedicated with diphenhydramine and steroids, with these drugs possibly tapered after demonstrating tolerance with the initial dosing.
The side effects related to wild-type EGFR binding are very similar to those of cetuximab, particularly acne-like rash and nail changes.25 Patients should be counselled on prophylactic skin measures, including barrier protection with alcohol-free moisturizing creams or ointments and sunlight avoidance strategies with protective clothing and broad-spectrum sunscreen. Reactive treatment measures for grade 1–2 rash include topical corticosteroids, systemic antibiotics (e.g. doxycycline, minocycline or cephalexin) to prevent bacterial superinfection, and continuation of topical moisturizers. Scalp rash may benefit from anti-inflammatory, antibacterial or antifungal shampoo. In the case of grade ≥3 rash, the addition of a short course of systemic steroids (e.g. prednisone 0.5 mg/kg for 7 days) can help resolve rash to grade ≤2. Providers should consider dermatology consultation in patients who develop rash or other skin toxicities of grade ≥3 or that do not improve within 2 weeks. For high-grade rash, treatment with low-dose acitretin or isotretinoin may be considered. Depending on the grade, treatment may be held.24
Pruritis is best managed with topical agents, such as steroids, calcineurin inhibitors or antipruritic agents. Higher-grade pruritis may benefit from oral antipruritic agents, oral corticosteroids, as well as gabapentin or pregabalin. Prophylaxis for paronychia includes measures such as skin irritant avoidance, nail moisturization and nail protection. Low-grade paronychia can improve with antimicrobial soaks and topical antiseptics with topical steroids or calcineurin inhibitors. High-grade paronychia may require the application of topical or oral antibacterial/antifungal agents. Dermatology and podiatry consults should be considered in severe or refractory cases.24 Oedema and hypoalbuminemia are common side effects as well, as seen with other c-MET inhibitors.25,38 This is generally mild and can be managed with compression hose and gentle diuresis with furosemide and spironolactone.
Comparisons to other current and investigational treatments for EGFR ex20ins non-small cell lung cancer
In addition to the bispecific antibody amivantamab, a selection of small-molecule TKIs are under investigation in EGFR ex20ins-mutated NSCLC (Table 1). Mobocertinib is a novel TKI designed to target EGFR ex20ins that has demonstrated promising activity in preclinical studies and is currently in clinical trials.39 Preliminary results from a phase I/II study of mobocertinib in treating EGFR ex20ins NSCLC found an ORR of 56% in patients without brain metastasis at baseline, but a decreased ORR of 25% in patients with brain metastasis.40,41 In the most recently published cohort of 114 platinum pre-treated patients with EGFR ex20ins tumours, confirmed ORR was 26% with median DOR of 17.5 months and median PFS of 7.3 months.42 Similarly to other anti-EGFR therapies, mobocertinib 160 mg had significant toxicities and was associated with diarrhoea in 90% of patients (including grade ≥3 diarrhoea in 22%), rash in 45% of patients, and required treatment discontinuation in 17% of patients.42 Neither amivantamab nor mobocertinib appear to have significant central nervous system penetration, which are limitations compared with osimertinib for common EGFR mutations.43
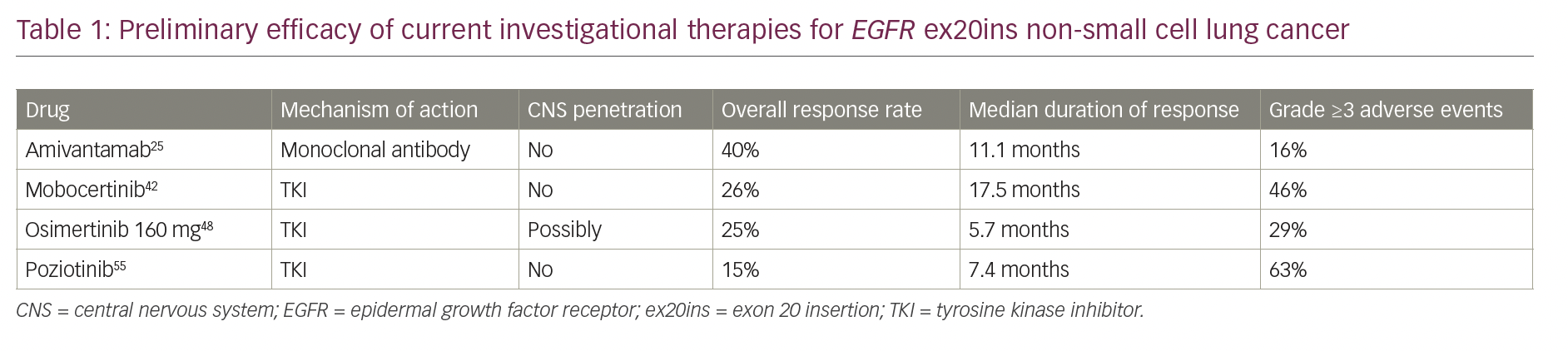
Osimertinib has been investigated in EGFR ex20ins tumours based on the excellent activity against classical TKI-sensitive EGFR mutations as well as preclinical data that it can bind ex20ins-mutated EGFR despite the presence of the rigid C-helix that sterically hinders the binding of first- and second-generation EGFR TKIs.44,45 The standard dose of osimertinib, 80 mg in EGFR ex20ins NSCLC, was investigated in two small single-arm clinical trials, both reporting no objective responses (ORR 0%).19,46 In tumours with classical EGFR mutations and leptomeningeal disease, the BLOOM study demonstrated that a higher dose of osimertinib 160 mg provided clinically meaningful benefit and was generally well tolerated.47 Following this, a phase II study of patients with EGFR ex20ins NSCLC treated with osimertinib 160 mg reported a confirmed ORR of 25% (5 of 20 patients including one complete response), and an impressive disease control rate of 85% with a median PFS of 9.7 months.48 The increased dose was well tolerated, and reported toxicities were similar to those expected with osimertinib, most prominently diarrhoea, followed by fatigue, thrombocytopaenia and rash.48 These results merit further study of whether dose escalation for osimertinib can improve outcomes in patients with EGFR ex20ins in NSCLC.
Poziotinib is a novel EGFR inhibitor that was designed as a smaller molecule than current first-generation EGFR TKIs, such as afatinib, allowing it to bypass the steric hindrance induced by ex20ins mutations.49 An initial phase II study of poziotinib in EGFR ex20ins NSCLC yielded a 44% ORR and a median PFS of 5.5 months.50 However, the follow-up multicentre ZENITH20 study demonstrated a lower ORR of 15% with poziotinib treatment for pre-treated ex20ins-mutated tumours with a median PFS of 4.2 months among 115 patients.51 Poziotinib also has considerable off-target EGFR wild-type activity, contributing to the high rate of treatment-related AEs identified in both clinical trials, including rash and diarrhoea, with frequent dose reductions or discontinuation.50,51 Alternative dosing regimens are being studied to overcome the substantial toxicity.52 Other drugs targeting EGFR ex20ins are currently in development as well (including CLN-081 and BDTX-189).53,54
Limitations and resistance to amivantamab
Amivantamab was designed to overcome resistance mechanisms to anti-EGFR TKIs through its bispecific antibody construct with extracellular binding of EGFR and dual engagement of MET. Though the clinical efficacy in both EGFR ex20ins and TKI resistance mutations is encouraging, only a subset of patients responds to amivantamab. Importantly, as a monoclonal antibody, amivantamab is expected to have poor blood–brain barrier penetration,55 and so its use may be limited in patients with intracranial metastases. Additionally, many patients have developed progressive disease on amivantamab therapy,3,4 highlighting the need to further explore the underpinnings of sensitivity and acquired resistance. Future studies and analyses will help determine the relative sensitivities of different exon 20 mutations to amivantamab and identify potential biomarkers predictive of response.
Current and future trials of amivantamab in NSCLC
Several trials of amivantamab are ongoing to investigate its potential for the treatment of NSCLC (Table 2). Part 2 of the CHRYSALIS study continues and will elucidate safety and preliminary efficacy for amivantamab in several cohorts, including EGFR ex20ins, classically EGFR-mutated NSCLC following progression on a third-generation EGFR TKI, classically EGFR-mutated NSCLC with documented C797S mutation or secondary MET amplification, and NSCLC with MET exon 14 skipping alterations.35
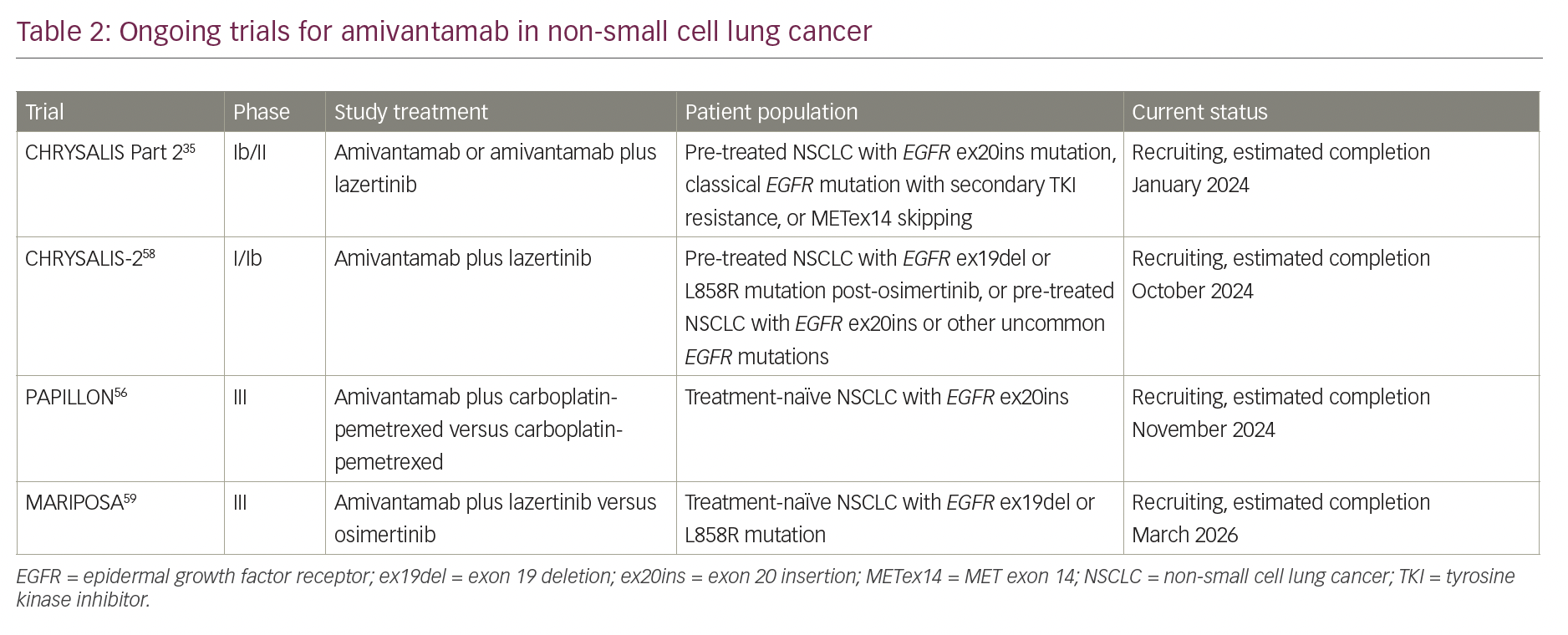
PAPILLON, a phase III study comparing first-line amivantamab plus carboplatin-pemetrexed with carboplatin-pemetrexed chemotherapy alone for advanced NSCLC, began in October 2020 and is currently recruiting patients with EGFR ex20ins mutations. The primary endpoint is PFS, and the study design includes optional cross-over to second-line amivantamab for patients in the chemotherapy only arm.56 PAPILLON thus seeks to understand if amivantamab can supplement first-line chemotherapy and extend PFS in patients with poor-risk EGFR ex20ins tumour mutations. Given the second-line response rates, it is unclear if targeted therapy alone with single-agent amivantamab could eventually replace chemotherapy for treatment-naïve patients, but subsequent studies may investigate this possibility. Other applications of amivantamab in EGFR ex20ins, such as use in the adjuvant setting, may be looked at in the future.
For patients with tumours harbouring EGFR mutations sensitive to TKIs, preclinical studies have found that lazertinib has higher selectivity, fewer off-target effects, and greater intracranial penetration than osimertinib,57 and the combination of amivantamab and lazertinib demonstrated efficacy in patients after relapse on osimertinib in a CHRYSALIS dose expansion arm.36 Next, the CHRYSALIS-2 trial seeks to study this combination in larger cohorts to prospectively validate biomarkers including response rates by different mechanisms of osimertinib resistance, and the study will also include pre-treated EGFR ex20ins and other uncommon EGFR mutation cohorts.58 Similarly, MARIPOSA, a phase III study of amivantamab and lazertinib combination therapy against osimertinib for NSCLC with EGFR exon 19 deletions or exon 21 L858R substitutions, began in September 2020 and is currently recruiting treatment-naïve patients.59 MARIPOSA thus seeks to demonstrate whether amivantamab and lazertinib combination therapy or lazertinib monotherapy can function as first-line therapies comparable to osimertinib monotherapy for EGFR-mutated NSCLC.
Conclusion
With its novel mechanism of action targeting dual EGFR and c-MET extracellular domains, the bispecific fully human antibody amivantamab has activity in both NSCLC with EGFR exon 20 insertion mutations and third-generation EGFR TKI-resistant NSCLC, demonstrated in various preclinical and clinical studies described above. The toxicity profile of amivantamab is favourable due to the bispecific nature of the antibody, making it a safe and viable therapeutic option for patients with both classical and non-classical EGFR mutations who have relapsed on standard first-line therapies. Compared with novel TKIs under investigation for EGFR ex20ins, including mobocertinib and poziotinib, amivantamab has demonstrated superior or equivalent response rates and significantly less toxicity in this population. Current and future studies evaluating amivantamab usage in combination therapies or as a first-line agent will be important and exciting to follow.