Radiation-induced esophagitis, caused by incidental damage to the mucosal lining of the esophagus during radiation therapy, is a common and clinically important toxicity in patients with lung cancer. Esophagitis generally develops 2–3 weeks after initiation of radiation therapy and presents as pain and dysphagia, which can negatively impact patient quality of life.1 Severe esophagitis requires parenteral nutrition and can lead to hospitalization and treatment interruptions to allow for healing.2,3 Therefore, strategies to prevent radiation-induced esophagitis are of paramount importance to increase tolerance to radiation therapy and enable continuation of treatment to improve tumor outcomes.
Reports of the incidence of esophagitis are inconsistent due to lack of standardized methods to measure esophagitis as well as differences in the treatment regimen utilized (i.e., sequential or concurrent chemotherapy, radiation technique, radiation dose, and volume of tissue irradiated).1,4,5 In patients treated with concurrent chemotherapy and radiation therapy, which is the current standard of care for patients with non-small cell lung cancer (NSCLC), severe esophagitis occurs in up to one-third of patients.6,7 Advanced radiation techniques and models to identify predictors of esophagitis are being developed with the goal of reducing the incidence and severity of esophagitis.5, 8–17 However, radiation-induced esophagitis remains an important limitation for radiation dose escalation efforts aimed at improving survival in patients with lung cancer.4 There is minimal utilization of radiation therapy alone for definitive treatment of lung cancer; therefore, this manuscript will focus on the current standard of care for patients with lung cancer, rather than chemotherapy and radiation therapy separately.
This review provides a general overview of radiation-induced esophagitis in patients with lung cancer and is not intended to be a comprehensive review of the literature. Rather, the literature was surveyed to provide a concise summary of current knowledge of the pathogenesis of radiation-induced esophagitis in patients with lung cancer and how it is measured, as well as an overview of models to predict the incidence of radiation-induced esophagitis and the current treatment landscape. Incidence of esophagitis in other disease states, for example metastases, and other radiation–drug interactions, such as tyrosine kinase inhibitors, which are rarely used during thoracic irradiation, are not included in the scope of this review.
Pathophysiology of mucositis
Radiation-induced esophagitis is much less studied than oral mucositis; however, the mucosa and the submucosa of the oral cavity and oropharynx are very similar to that of the esophagus up to the gastro-esophageal junction.18 Both are lined by keratinized and non-keratinized squamous epithelial cells overlying the lamina propria and the submucosa, which serve to protect underlying tissue. The esophagus has the same overall structure, but with muscularis mucosae between the lamina propria and the submucosa. As such, the pathophysiology of mucositis in both the oral cavity and esophagus is similar.
The current, generally accepted model outlining the five stages of mucositis—initiation, upregulation/activation, signal amplification, ulceration, and healing—was put forward by Sonis and colleagues and is relevant for both oral mucositis and esophagitis.19–22 In the initial phase, damage to epithelial cells occurs as a result of radiation therapy and/or chemotherapy during oncology treatment.20 Radiation induces double-stranded DNA breaks as a result of both direct damage to DNA and indirect damage caused by the generation of reactive oxygen species (ROS). In the second stage, release of ROS and other endogenous chemotherapy-related molecular patterns (CRAMPS) or damage-associated molecular patterns (DAMPs) from epithelial cells damaged in the first stage initiates a primary response that includes fibronectin breakdown, macrophage-mediated activation of matrix metalloproteinases, influx of inflammatory cells, and release of cytokines that further induce local tissue damage. Subsequently, a signal amplification stage occurs, which results in increased tissue damage and apoptosis through activation of nuclear factor (NF)-kβ, tumor necrosis factor (TNF)-α, and other cytokines, which results in a positive-feedback loop.
Among the cells damaged during these processes are the basal epithelial cells that generate the overlying mature squamous cells. The overlying squamous cells are more resistant to the effects of radiation therapy but continue to shed normally over time. The damage to the basal cells results in an insufficient supply of mature squamous cells, which in turn causes the surface of the epithelium to break down. The loss of the epithelial layer of mature squamous cells starts the next stage of the development of mucositis: ulceration and the loss of epithelia integrity. This gradual loss of the overlying cells without available replacement cells leads to a 2-week delay from the start of radiation therapy to when clinically evident mucositis sets in. The final stage of mucositis, healing, occurs spontaneously as a result of epithelial cell proliferation, differentiation, and migration from the wound margins in response to signaling from the extracellular matrix. During radiation therapy, the damaging insult is given daily, thus delaying the healing stage until after the radiation therapy is complete, with mucositis generally resolving 2–3 weeks after cessation of radiation therapy. There are currently no data available that directly correlate esophagitis with outcomes in patients with lung cancer, and so the detrimental effect of esophagitis on cure and control rates for these patients is unknown.
Measurement of esophagitis
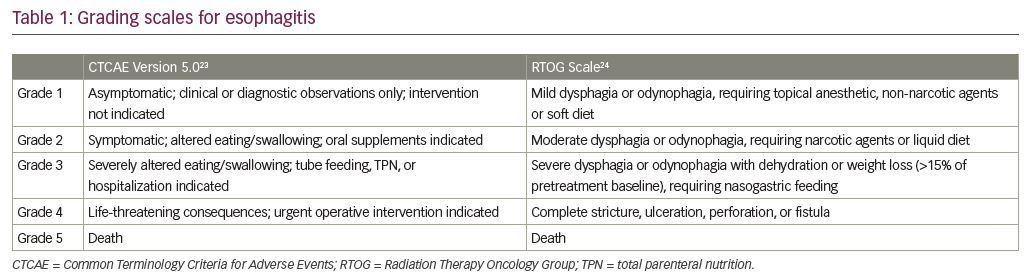
No validated, standard measurement for esophagitis exists. Currently, esophagitis is generally scored using subjective measures, which are imprecise by nature. The most common are the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE)23 and the Radiation Therapy Oncology Group (RTOG) Scale (Table 1).24 Both are observer rather than patient-scored scales and are arbitrary classifications for esophagitis. These grading scales do not represent true numerical values, and therefore scores cannot be added or averaged. Rather than 1 to 5, they could equally be called A to E, more appropriately showing that non-parametric statistics must be used with these measures.
A modification of the CTCAE is the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), designed to measure the patient experience of symptoms rather than proxy measurement by someone else.25 However, patients’ reports of the severity of symptoms of esophagitis are not expected to be uniform between patients and may not even be reproducible in the individual patient because many outside factors can influence the perception of esophagitis, such as fatigue or depression. For clinical research, the MD Anderson Symptom Inventory for Lung Cancer scale, which assesses general symptoms during the treatment of lung cancer (13 of which are common to tumors at all sites and 3 that are specific to lung cancer), has also been used to measure toxicity during radiation therapy, but this measure is not specific to esophagitis.26,27 The core symptoms are pain, fatigue (tiredness), nausea, disturbed sleep, distress, shortness of breath, difficulty remembering, lack of appetite, drowsiness, dry mouth, feeling sad, vomiting, and numbness or tingling; and the 3 added symptoms are coughing, constipation, and sore throat, each patient reported on a 0–10 scale. Applying these measures directly to esophagitis requires assuming that reports of pain (the primary clinical symptom of esophagitis) and perhaps nausea are due to esophagitis.
Clinical assessment of radiation-induced esophagitis, including ruling out other causes of esophagitis, such as candida and viral infection, often requires more objective measures. Objective measurement of esophagitis through direct observation of the esophagus requires multiple endoscopies. This is not a practical or accurate method of measuring acute esophagitis, and is not recommended. To replace direct observation, the ability to objectively measure esophagitis utilizing imaging techniques has been examined. Studies showed that measuring esophageal expansion or cross-sectional areas on serial computerized tomography (CT) scans during radiation therapy can be used to quantify radiation-induced injury and correlated with the subjective measurement of esophagitis.28 Positron emission tomography (PET) scanning has also been examined for a potential correlation with subjective grades of esophagitis. In one study, the increase in standardized uptake values at 2 weeks correlated with grade 3 esophagitis.14 Another study found fluorodeoxyglucose (FDG) uptake was highly correlated with esophagitis grade.29
Predictors of esophagitis during radiation therapy
Esophagitis occurs during radiation therapy because part of the esophagus is incidentally irradiated during treatment. Tumor location adjacent to the esophagus or mediastinal lymph node involvement increases the risk of esophageal mucosa falling within the radiation field. The dose of radiation therapy that a structure, such as the esophagus, receives is reported as a dose-volume histogram. During treatment planning, the dose to the esophagus along its course is calculated as the percentage of the esophagus that will receive a specified dose and is reported as: Ddose = volume (%). For example, D30 = 50 states that 50% of the esophagus receives 30 Gy of radiation.
A number of studies have examined predictors of radiation-induced esophagitis, and have linked female sex, low body mass index, pretreatment dysphagia, higher nodal stage, and concurrent chemotherapy to higher incidence of esophagitis.1,5,7,9,11,12,30,31 The impact of radiation technique and dosimetric factors on the incidence of esophagitis has also been examined. For example, a 2012 study by Gomez and colleagues on predictors of high-grade esophagitis after different techniques of radiation therapy (3D-conformal radiation therapy [CRT], intensity-modulated radiation therapy [IMRT], and proton beam therapy) for NSCLC found the incidence of severe esophagitis (grade ≥3 using the CTCAE scale) correlated more closely with maximum esophagus dose than with the mean dose to the esophagus, highlighting the importance of weighing the percentage of esophagus receiving a high dose when evaluating treatment plans.7 The study also found the incidence of grade ≥3 esophagitis was highest with IMRT (28% versus 8% [3D-CRT] and 6% [proton beam therapy]), although other studies have shown similar incidence of esophagitis with IMRT and 3D-CRT.6,7,32,33 A study by Huang et al., modeling the relationship between esophagus dose-volume parameters and risk of grade ≥2 esophagitis, found that the percent of the esophagus receiving at least 40 Gy was more closely correlated with the rate of toxicity than the mean dose.34 In contrast, in patients treated with low-modulated IMRT, the mean esophageal dose correlated well with toxicity, with a mean esophageal dose of 15 Gy correlated with a 50% risk of grade 1 esophagitis (measured using the older RTOG scale) and a mean dose of 46 Gy correlated with a 50% risk of grade 2 esophagitis.35
Attempts to identify radiation technique and dosimetry-related factors predictive for esophagitis have failed to establish consensus on the optimal dose thresholds or relative importance of different predictors.5,7,12,34,35 Limited sensitivity of measurements to assess esophagitis and the use of different endpoints in different studies (i.e., CTCAE versus RTOG) complicate comparison between studies. In addition, most models to date examining predictors of esophagitis have looked at the incidence of grade ≥2 esophagitis, due to the low risk of grade ≥3 events. A recent study of 202 consecutive patients treated for NSCLC with radiation therapy utilized 35 clinical factors in a multivariate analysis performed by 11 machine-learning algorithms in an attempt to validate and rank predictors of grade ≥3 esophagitis.12 The rate of grade ≥3 esophagitis was 11% in the study; however, no reliable predictive models for severe esophagitis were identified. Additional studies are needed to establish reliable and consistent predictors for grade ≥3 esophagitis to facilitate identification of patients most at risk for this toxicity, and to aid development of treatment strategies to mitigate radiation-induced esophagitis.
Treatment of radiation-induced esophagitis
Currently, no standard method for preventing or treating radiation-induced esophagitis exists. Although the Multinational Associate of Supportive Care in Cancer and the International Society of Oral Oncology have published joint, evidence-based recommendations for the management of mucositis secondary to cancer therapy, most of these apply to oral mucositis.36 For example, they cite strong evidence for the treatment of radiation-induced mucositis with benzydamine mouthwash, and weaker evidence supporting the use of treatments such as low-level laser therapy, morphine mouth rinses, doxepin mouth rinses, and zinc supplements to prevent radiation-induced mucositis. However, there are no studies supporting the use of these treatments for patients with esophagitis, and they would be technically difficult to implement to treat the esophagus.
Several compounds have been evaluated in clinical trials for the prevention or treatment of radiation-induced esophagitis, with conflicting results. In early studies, amifostine, a scavenger of free radicals produced during radiation therapy, showed mixed results as a potential radioprotective agent;37–41 however, no reduction in severe esophagitis was observed in a phase III study in patients with NSCLC on chemoradiotherapy (30% incidence of grade ≥3 esophagitis based on CTCAE criteria with amifostine versus 34% with control, p=0.9).42 Sucralfate, a treatment for gastroduodenal ulcers, provided significant relief of esophagitis symptoms within 7 days (80% versus 10%) and accelerated tumor healing compared with treatment with the antacid sodium alginate in patients with esophageal cancer treated with radiation therapy.43 However, a separate trial found no substantial benefit of sucralfate versus placebo with regard to esophagitis and increased gastrointestinal toxicity (58% of sucralfate-treated patients versus 14% of placebo patients; p>0.0001) and study drug discontinuation (40% for sucralfate and 4% for placebo) in the sucralfate group.44 Sodium alginate has also been studied as a potential treatment for esophagitis in patients treated with chemoradiotherapy for NSCLC.45 Rates of severe esophagitis (CTCAE grade ≥3) were 12.8% in patients treated with sodium alginate at the start of chemoradiotherapy, 9.8% in patients treated with sodium alginate at onset of esophagitis, and 19.4% in the control group; however, differences were not statistically significant.
Several natural products have been evaluated in small, randomized, clinical trials for the prevention of radiation-induced esophagitis. Multiple studies have shown that honey can reduce radiation-induced oral mucositis suggesting a potential therapeutic role for patients with esophagitis as well.46 However, a randomized trial of Manuka honey (liquid or lozenges) versus supportive care found no difference in patient-reported pain with swallowing (numerical rating pain scale) at 4 weeks in patients receiving chemoradiotherapy for NSCLC.47 Whether an effect might be found using different treatment protocols or sources of honey remains to be determined. Studies with epigallocatechin-3-gallate (EGCG), a compound extracted from green tea, have shown promise for the treatment of esophagitis. In an open-label, phase II study in patients with esophageal cancer receiving chemoradiotherapy or definitive radiation therapy, RTOG esophagitis scores decreased after EGCG treatment.48 Further, in a trial of 83 patients being treated for lung cancer, both prophylactic use of EGCG (at the start of chemoradiotherapy) and therapeutic application (at onset of esophagitis) resulted in a reduction in maximum acute esophagitis grade versus standard of care, with a slightly better effect with prophylactic application.49
Oral glutamine, which may play a role in tissue healing in response to damage from chemotherapy and radiation, has been shown to significantly reduce the severity of oral mucositis in patients treated with chemoradiotherapy for head and neck, suggesting the potential for a similar effect for esophagitis.50–52 In a single-center, randomized trial of 60 patients treated with chemotherapy and radiation therapy for NSCLC, glutamine treatment was associated with significantly less grade 2/3 esophagitis (6.7% versus 53.3%) and delayed grade 2/3 onset (median 18.2 days versus 12.4 days) compared with the control arm.53 The Chinese medicine Zhuye Shigao Granules has also been shown to significantly reduce the incidence of grade 2 esophagitis (9% versus 23%) in patients receiving concurrent or sequential chemoradiotherapy for lung, esophagus, or mediastinal cancer.54 Finally, whole course nutrition management, utilizing a complex nutritional plan based on a detailed nutritional assessment of the patient with diet monitored and adjusted as needed throughout treatment, significantly reduced maximum esophagitis grade ≥2 (20% in the nutritional management arm and 43% in the control arm).55
In addition to drug- and nutritional-based interventions to treat esophagitis, strategies to reduce incidental irradiation of the esophagus have been explored. However, in a randomized trial comparing target volume reduction informed by 18F-FDG PET compared with conventional targeting, the rate of grade ≥3 esophagitis or dysphagia was 16% in both arms.15
Several active trials on ClinicalTrials.gov (as of April 15, 2020) for the prevention of esophagitis during chemotherapy and radiation therapy for lung cancer are ongoing. These include additional studies testing EGCG and oral nutritional supplements. Novel agents being tested include mecapegfilgrastim, a biosimilar to recombinant human granulocyte-colony stimulating factor (rhG-CSF), and avasopasem manganese (GC4419), a small molecule superoxide dismutase mimetic shown to reduce the duration, severity, and incidence of radiation-induced severe oral mucositis in patients with head and neck cancer undergoing radiation therapy.56
Conclusion
Despite advances in radiation therapy, esophagitis remains an important clinical concern in patients treated with concurrent chemotherapy and radiation therapy for lung malignancies. Dose escalation efforts to improve survival rates in patients with lung cancer have been limited by this and other radiation-induced toxicities. Efforts to improve and standardize objective measurement of esophagitis and development of predictive models to inform risk will be beneficial in developing strategies to mitigate the risk of severe esophagitis. Development of effective treatments for radiation-induced esophagitis could allow the use of higher doses of radiation when treating patients with lung cancer, potentially increasing cure rates.