Somatic DNA mutational analysis has transformed how we characterize existing cancer and select treatments. For example, circulating tumour cell-free DNA (cfDNA) can be used to select targeted therapies, monitor treatment response and detect disease recurrence.1 However, early attempts with targeted sequencing of circulating tumour cfDNA to screen for multiple cancers were unsuccessful because of low sensitivity and specificity.2,3
Currently, cancer screening is performed one organ at a time using imaging tests or histopathological analyses of biopsied tissue or other biospecimens. In the United States, only five types of cancer are recommended for screening in the general population: colon, breast, cervical, lung (in a high-risk population) and prostate (on an individualized basis).4–8 There are several reasons why this single-cancer screening paradigm is failing the population and not detecting a sufficient number of cancers. First, unscreened cancers account for more than 60% of all cancer diagnoses and approximately 71% of cancer deaths (Figure 1A).9 (Data on file; calculated based on Surveillance, Epidemiology, and End Results Program and Cancer Statistics).10 Second, single-cancer tests have optimized sensitivity, and accept high false-positive rates for each cancer. For an individual receiving all the recommended single-cancer screening tests in a single year, the cumulative false-positive rate for men is 31% (including colorectal, lung and prostate cancer) and for women is 43% (including breast, cervical, colorectal and lung cancer) (Figure 1B).11–15 Finally, any individual undergoing one of these single-cancer screening tests is much more likely to be diagnosed with a different cancer in that year (Figure 1C).16 For example, in the populations being screened for cervical, colorectal or breast cancer, the annual incidence rate of other cancers is 2–24-fold higher than that of the single cancer being screened.16
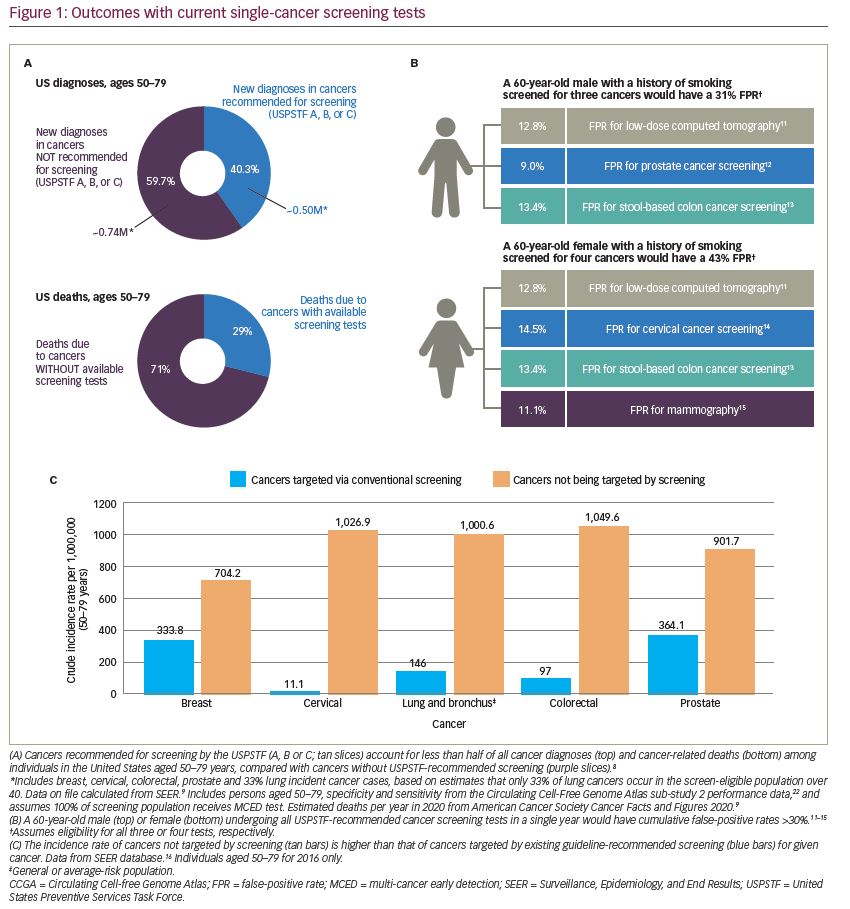
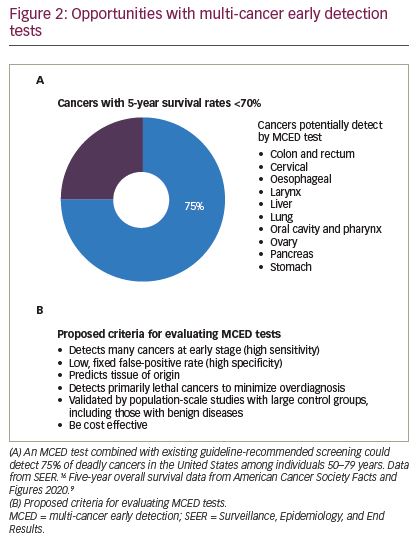
For these reasons, the opportunity for early detection of multiple cancers with a single test is potentially transformational (Figure 2A).9,17 A conceptual shift is required, from screening only for guideline-recommended individual cancers to screening individuals for any cancer in combination with existing guideline recommendations. Because the occurrence of individual cancers in the general population is low, the number of people who need to be screened to find a single cancer is very large. However, this number decreases when multiple types of cancer are screened for in aggregate, thereby increasing the aggregate prevalence and resulting in a higher overall positive predictive value (PPV).18 The transformational idea of a multi-cancer early detection (MCED) test is the ability to detect multiple cancers and dramatically increase the cancer detection rate (CDR) in the population, i.e. the number of cancers detected divided by the number of cancers expected in the population monitored. In the United States, for example, there will be an estimated 1.2 million cases of cancer in 2021 in adults aged 50–79 years; mammography is expected to detect 114,000 of those cancers, for a CDR of approximately 9%.17 Even when all five single-cancer screening tests are combined, the CDR is still only approximately 16%.17,19,20 Although this is a notable accomplishment, by itself it is evident that it is not enough, as other cancers comprise approximately 51% of cancers diagnosed and 56% of cancer deaths.21 Importantly, MCED tests are intended to complement, not replace, single-cancer screening tests by being used in combination. Because an MCED test could maximize overall cancer detection in the population while minimizing harms with its low fixed false-positive rate, it could translate into higher value and a greater public health impact compared with single-cancer screening tests alone.
As it relates to minimizing harms, a false-positive rate of <1% associated with a multi-cancer test, compared with a higher cumulative false-positive rate associated with multiple single-cancer tests, could result in reduced patient anxiety, fewer unnecessary diagnostic workups, and lower healthcare resource use and costs. In a health economic model comparing the clinical outcomes of adding an MCED test to current cancer screening with current screening alone, the addition of an annual MCED test was predicted to provide an incremental gain of 0.34 quality-adjusted life-years per person, and was a highly cost-effective intervention.23 Of note, the current PATHFINDER study (ClinicalTrials.gov Identifier: NCT04241796) will provide patient-reported outcomes among patients who utilize a multi-cancer detection test in a clinical practice setting. Additionally, a multi-cancer test could offer the unique ability to detect any cancer, versus a traditional organ-specific test that only detects a single cancer. This could be especially valuable in detecting lethal cancers beyond the ones detected with traditional organ-specific screening paradigms. The inclusion of cancer signal origin (i.e. tissue of origin) analysis in a multi-cancer detection test is an important component to reducing patient anxiety and streamlining the diagnostic evaluation.24
In terms of estimating the potential public health impact, recent modelling efforts show that the implementation of an MCED test in addition to usual care (e.g. screening, incidental detection, symptomatic workup) may result in earlier cancer detection for 485 cancers per year per 100,000 individuals compared with usual care alone. For those with cancers detected earlier, this is expected to translate to a 78% reduction in late-stage cancer incidence, a 39% reduction in 5-year cancer mortality, and ultimately a 26% reduction of all cancer-related deaths.25 It is well recognized that earlier stage-shifting of cancer diagnosis, one of the primary goals of an MCED, is strongly linked with higher rates of survival at 5 years.26 The annual statistics published by the American Cancer Society also note that 5-year survival rates are highest for many cancers when diagnosis occurs at earlier stages.21
Indeed, a new generation of cfDNA-based MCED tests is being developed and will be available for clinical use in the near future.27 Several analytical strategies have been proposed, including analysis of cfDNA and protein biomarkers,28 cfDNA fragmentation patterns,29 and DNA methylation patterns.22,30 It should be noted that the tumour-type specific sensitivities of cfDNA-based MCED tests are dependent on the amount of tumour cfDNA shed into the blood, which varies for different tumour types and cancer stages.31 These methods report simultaneous detection of signals from 7 to more than 50 types of cancer, with high specificity (each approach reports ≥95%) and moderate-to-high sensitivities (ranging from 44% to 98%, dependent upon cancer type and stage distribution), as well as prediction of the cancer signal origin with varying degrees of accuracy (61–93%).32 In addition, a few large prospective studies, such as DETECT-A (~10,000 participants), STRIVE (ClinicalTrials.gov Identifier: NCT03085888; ~100,000 participants), SUMMIT (ClinicalTrials.gov Identifier: NCT03934866; ~25,000 participants), PATHFINDER (NCT04241796; ~6,200 participants) and ASCEND (ClinicalTrials.gov Identifier: NCT04213326; 3,000 participants), are completed or are under way to clinically validate MCED tests.27,33 Due to the novelty of genomic MCED tests, there is not an accepted framework for how such tests should be evaluated. Although some criteria have been suggested to evaluate the performance and safety, work needs to be done to clarify analytical and clinical validity, benefit–risk, and clinical utility of MCED test.18,27,28 As shown in Figure 2B, we propose that a cfDNA-based MCED test to be used alongside existing guideline-recommended screening should:
- Target a population group with an elevated risk of cancer to enhance PPV. For early cancer detection, age is the most important risk factor34 – the incidence of cancer in individuals older than 50 years is 12 times higher than that in younger individuals.16 Importantly, in this group, when all five guideline-recommended single-cancer screening tests are combined, the CDR is only approximately 16% of all incident cancers. There are other populations with elevated risk for many cancers, such as smokers, cancer survivors and those with diabetes and obesity, but analyses are forthcoming to better quantify these risks, as most studies have evaluated risks for single cancers. Limiting screening only to individuals with a high risk of cancer due to germline mutations or lifestyle choices (e.g. smoking) may be too stringent for population health benefit.18
- Detect as many types of cancer as possible to maximize cancer detection in the overall population, in an effort to detect cancers early when there is the best chance for cure or effective therapy.17,18
- Have a low, fixed false-positive rate to allow additional cancer types to be detected by the MCED test without increasing the number of false positives. By contrast, screening with multiple single-cancer detection tests would result in a cumulative false-positive rate much higher than that from their respective individual tests.35
- Be able to accurately localize the cancer signal and predict the cancer signal origin to direct a focused and efficient evaluation and help reduce the ‘diagnostic odyssey’ that many patients experience.18,28 In fact, a recent expert review stated that this was a requirement for an MCED test.24
- Potentially limit detection to cancers that are most deadly during the typical human lifespan to minimize overdiagnosis of indolent tumours that would not otherwise be fatal (i.e. tumours that people die with, but not from).18,27 Because rapidly growing, invasive tumours tend to shed more DNA into blood than slower growing, non-invasive tumours, cfDNA MCED tests may preferentially detect tumours that are more likely to be lethal.22,32
- Balance sensitivity and specificity to optimize cancer detection while minimizing harms from false positives. The current single-cancer screening paradigm values sensitivity over specificity in an effort to detect as many cancers as possible, but this can result in overdiagnosis.28 Because specificity and disease prevalence influence PPV more than sensitivity,18 an MCED test with only moderate sensitivity but very high specificity and aggregate prevalence could detect more cancers than a single-cancer test with very high sensitivity but lower specificity and disease prevalence.
- Be validated by prospective, multicentre, population-scale studies with longitudinal follow-up.18 Although a reduction in cancer-specific mortality is generally the ideal endpoint in cancer screening trials, single-cancer studies have proven difficult to conduct, often required more than a decade of follow-up to draw conclusions, and have been largely government-funded. A mortality study for an MCED test is neither practical nor feasible to execute within an optimal timeframe, and could significantly delay clinical implementation.36,37 Detecting statistically significant reductions in mortality for the majority of cancers could require more than a million participants to be enrolled and followed for a decade or more,36 during which time many cancers will occur and many lives will be lost to late-stage diagnosis. Instead, analytical models have been developed to evaluate the effects of early cancer detection in hypothetical populations,38 which have supported the deployment of non-invasive cancer screening methods. Test performance measures (e.g. very high specificity to maximize PPV) validated at the population level should be sufficient to support clinical use while more data, such as real-world evidence (e.g. through registries or administrative database analysis), are collected.
- Be evaluated in a study with a large number of control individuals without cancer, including those with benign neoplasms and inflammatory conditions, to ensure that test results are highly specific.28
- Be cost effective,18,27 providing high value relative to potential harms and costs.
For any MCED test, a high PPV is of paramount importance to minimize false positives and their accompanying physical, psychological and economic burden. In a population with a 1.3% incidence rate per year of cancer, an MCED test with a sensitivity of 55% and a specificity of 99.3% could detect an estimated 715 cancers per 100,000 screened persons with 691 false positives, corresponding to a PPV of 51%.22 By contrast, the published PPVs for existing breast and lung cancer screening tests are approximately 4% each.15,39 It should also be noted that the new MCED tests under development are expected to meet the basic clinical and analytical standards in accordance with regulatory requirements24,40,41 and the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines.42
MCED tests could transform the cancer detection landscape; however, integrating this new technology into our healthcare system will require thoughtful benefit–risk assessments.18,24,27 To do this, we need stakeholders to identify a sensible framework for evaluating the benefits and risks associated with an MCED test that is both rigorous and pragmatic to ensure that patients have access to technology that has been validated through standardized and clear criteria,9,42 and may soon be available. Together, this could begin to impact the trajectory of cancer mortality, which is soon to be the world’s number one killer.43