Melanoma represents 4% of all malignant tumors of the skin yet is responsible for 80% of deaths from skin cancer; it was estimated that 9,180 people would die of melanoma in 2012.1 The disease disproportionately targets young people: from 2005 to 2009 the age-adjusted incidence rate of melanoma in the US was 21.0 per 100,000 men and women per year, 58.5% of whom were below the age of 64.1 The number of new cases of melanoma in the US has been increasing for the last 30 years: between 1985 and 2009, the incidence of melanoma in the US has more than doubled.1 Treatment of early-stage melanoma by surgery can be curative, but patients with locally advanced disease have a high risk for recurrence and death. Localized melanoma has a 98.2% 5-year survival rate; however, if the cancer has metastasized the 5-year survival rate is 15.1%.1 The American Joint Committee on Cancer (AJCC) classification is the most widely accepted staging system for melanoma and has been recently updated.2
Despite considerable research, the treatment of advanced disease remains challenging. Historically, the alkylating agent, dacarbazine (DTIC), has been the standard therapy for patients with metastatic melanoma. High-dose interleukin-2 (HD IL-2) has been shown to achieve durable long-term complete responses in a small proportion of patients.3 In 2011, the US Food and Drug Administration (FDA) granted approval for ipilimumab (an anti-cytotoxic T-lymphocyte antigen 4 [CTLA-4] human monoclonal antibody) and vemurafenib (an inhibitor of proto-oncogene B-Raf [BRAF] kinase); in 2013, dabrafenib and trametinib were approved for the management of stage IV disease harboring BRAF mutations.
The current treatment for melanoma with lymph node involvement, but without distant metastasis, is surgical excision and lymph node dissection. However, the risk for recurrence of melanoma after surgery is reported to be approximately 60% for stage IIB patients and 75% for stage III patients.4 The probability of recurrence is defined as low, intermediate, or high risk depending on the thickness of the primary tumor, the presence of ulceration or mitoses in the primary tumor, and the presence of nodal or in-transit or satellite metastases around the primary lesion.5,6
Adjuvant therapy is offered after surgical treatment has removed all detectable disease and is given with the intent of reducing relapse risk due to occult disease. Adjuvant therapy should be considered for patients whose risk for recurrence exceeds 30%, i.e. patients with either stage IIB melanoma with a primary thickness greater than 4 mm or greater than 2 mm with ulceration or stage III melanoma.4 For patients with stage I–II disease, sentinel node mapping, a procedure that identifies micro-metastatic disease in the regional lymph nodes with greater precision than an elective lymph node dissection, may be used to select patients for adjuvant therapy.7
This article will discuss the use of IFN-a in the adjuvant setting and will outline other evolving options including vaccines, CTLA-4 blockade, chemotherapy, and radiotherapy.
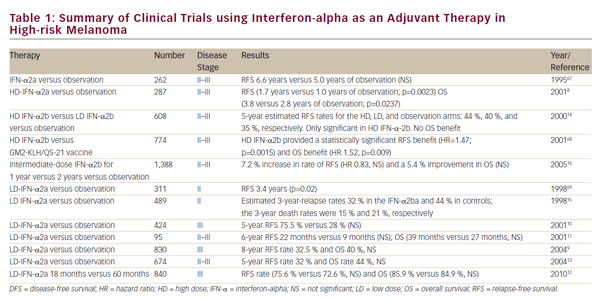
Interferon Adjuvant Therapy in Melanoma HD interferon-alpha (HD-IFN-a) is the only FDA-approved agent for adjuvant therapy for high-risk melanoma and has shown clinical efficacy; however, the optimal dosing and duration of treatment are still unclear. The schedule for administration of the therapy is as follows: induction: IFN-a: 20 MU, intravenously (IV) 5 days a week for 4 weeks; followed by maintenance: IFN-a: 10 MU, subcutaneously (SC) three times a week for 48 weeks. The FDA approved the use of adjuvant HD-IFN-a in high-risk melanoma after a pivotal phase III trial, E1684, demonstrated significant reduction of recurrence and mortality in patients with high-risk (stage IIB and IIIA/B) melanoma.8 Treatment with HD-IFN-a prolonged the relapsefree survival (RFS) at 5 years by 40%, and overall survival (OS) by 28% compared with the observation group. Since the aim of the study was to administer maximally tolerated doses of IFN, toxicity was significant among those treated with HD-IFN-a. Grade III toxicity occurred in 67% of patients, grade IV toxicity in 9%, and there were two early deaths secondary to hepatotoxicity. This prompted the investigation of the efficacy of lower doses of IFN-a.
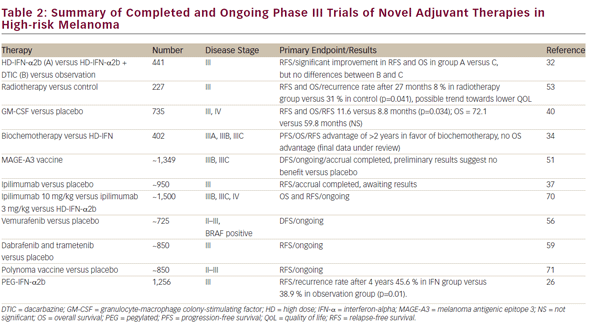
A number of alternative IFN-a regimens have been studied with varying degrees of success and are summarized in Table 1. Randomized controlled trials have utilized very low dose (LD) (1 MU every other day),9 and LD (≤3 MU/dose),10–14 but these did not demonstrate significant clinical benefit. A trial comparing LD- and HD-IFN-a showed improvements in RFS in the high-dose setting, but not with low doses.14 A French trial reported significant benefits of treatment with LD-IFN-a for 18 months in terms of OS and RFS,15 but this was in stage II patients before clinically detectable lymph node metastases had developed. Given the relatively good prognosis of these patients, the cost–benefit advantages of this approach are questionable. An intermediate dose failed to demonstrate significant benefits in terms of OS or RFS.16
The duration of therapy of HD-IFN-a has been the subject of debate. In a study to determine the potential benefit of adding a short-term highdose induction phase to conventional adjuvant LD-IFN-a treatment of patients with high-risk primary melanoma, no significant differences were observed in terms of OS, or RFS when compared with a conventionally treated group of patients.17 The Hellenic group found no differences in RFS and OS with 1 month versus 1 year of adjuvant HD-IFN-a therapy in patients with resected, high-risk (stage IIB to III) melanoma.18 However, this trial was underpowered to achieve statistical significance. Another trial compared therapy for 1 month with therapy for 1 year for patients with intermediate-risk melanoma. The trial was stopped for futility, because the 1-month HD-IFN-a was not better than observation. It was concluded that 1 month of therapy with HD-IFN-a is insufficient to improve RFS and OS.19 Further efforts to modify the IFN-a dose or schedule have not improved its efficacy.20 These data suggest that the benefit of IFN-a requires more lengthy treatment than just the induction phase of the high-dose regimen. The optimal dosing and duration of treatment with IFN-a could be improved with further study, but the promise of newer agents suggests other approaches.
In a meta-analysis, no optimal IFN-a dose and/or treatment duration or a subset of patients more responsive to adjuvant therapy was identified.21 The meta-analysis of 14 studies concluded that IFN-a treatment was associated with a statistically significant improvement in RFS in 10 of the 17 comparisons (hazard ratio [HR] for disease recurrence = 0.82, 95% confidence interval [CI] = 0.77–0.87; p<0.001) and improved OS in four of the 14 comparisons (HR for death = 0.89, 95% CI=0.83–0.96; p=0.002). These three meta-analyses and one systematic review to date have found that IFN-a treatment was associated with a statistically significant improvement RFS with a lesser impact on OS, and there is no evidence of survival gain beyond 10 years.21–24 More recently, a Cochrane review of 18 RCTs and 10,499 patients showed a RFS benefit (HR 0.83; p 0.0001) and OS benefit (HR 0.91; p=0.003).25
In 2011, the FDA-approved pegylated IFN-a-2b (PEG-IFN-a-2b) for the adjuvant treatment of patients with high-risk melanoma, following the results of a phase III trial.26 Long-term (7.6 years) follow-up data showed improved RFS but not OS in the treatment arm.27 Subgroup analysis suggests that the benefit of PEG-IFN may be limited to patients with micrometastatic disease and ulcerated primaries.27 A recent phase II trial compared PEG-IFN-a-2b with LD-IFN-a-2b in patients with resected stage IIA–IIIB melanoma. No differences were observed between the two groups in terms of RFS, OS, or distant metastasis-free survival (DMFS), although PEG-IFN-a was associated with higher rates of grade 3–4 adverse events (47.3% versus 25.2%) and discontinuations (54.3% versus 30.4%) compared with LD-IFN-a.28 PEG-IFN-a may be considered as an alternative to HD-IFN-a for patients unwilling to undergo a high-dose regimen.
In summary, in the clinical trials of IFN-a adjuvant therapy to date, only HD-IFN-a has consistently shown an improvement of RFS in all studies and has shown an increase in OS in randomized clinical trials (RCTs). However, it remains uncertain which patient population benefits most from adjuvant treatment. In addition, the use of IFN-a is associated with toxicity and requires a team with experience in its management. There is a need for other therapeutic regimes in adjuvant treatment of melanoma.
Current Research into Alternative Adjuvant Therapies
A number of alternative adjuvant therapies are being investigated. A summary is given in Table 2.
Biochemotherapy
Several trials have evaluated the use of adjuvant chemotherapy following surgical resection in high-risk patients, but none have demonstrated significant benefits in phase III clinical trials.29 Biochemotherapy (the combination of biologics and chemotherapy) has demonstrated improvements in RFS in an RCT30 and is associated with long-term survival as demonstrated by a single-institution series.31 This has led to investigation of its use in the adjuvant setting.
A phase III trial compared a combination of LD-IFN-a with DTIC and LDIFN- a alone following lymph node dissection. However, the addition of DTIC reversed the beneficial effect of adjuvant IFN-a therapy.32 A recent study aimed to determine whether a short course of biochemotherapy would be more effective than HD-IFN-a as adjuvant treatment in patients with high-risk melanoma (stage IIIB and IV patients). A study comparing HD IFN-a, LD IFN-a, and biochemotherapy (consisting of DTIC, cisplatin, vinblastine, IL-2, and IFN-a) in patients with stage III disease was unable to show a statistically significant improvement in patients receiving biochemotherapy compared with HD IFN-a or ID IFN-a.33 However, this study aimed to show a doubling of RFS and OS with biochemotherapy over IFN-a. A less-ambitious target increase in RFS may have yielded different results. Furthermore, the study stopped early due to slow accrual with only 138 of the planned 200 patients enrolled, further limiting the power of the study. A later study including a larger number of patients, also with stage III disease, revealed biochemotherapy (consisting of DTIC, cisplatin, vinblastine, IL-2, IFN-a, and granulocyte colony-stimulating factor [G-CSF]) produced a statistically significant improvement in RFS (4.31 versus 1.9 year median) compared with HD IFN-a, but no discernable difference in OS.34
These data are intriguing and support the use of biochemotherapy in the adjuvant setting for patients ineligible for clinical trials or for patients not wishing to participate in clinical studies and who have access to medical centers with expertise in the administration of this complex regimen. Unfortunately, the toxicity and complexity of the administration of this regimen is a significant barrier to making it standard practice for stage III melanoma, and make it ill-suited for a control arm in large randomized controlled trials.
Checkpoint Blockade
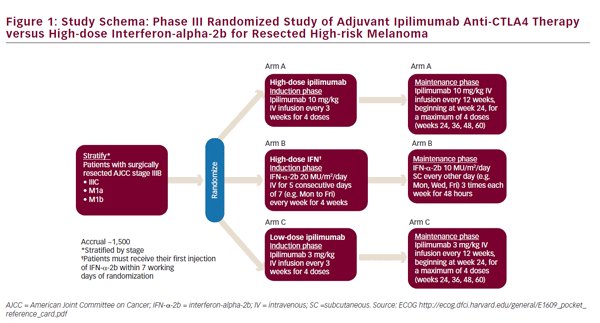
The use of ipilimumab as first-line treatment is well-established,35 and clinical trials are now underway to assess its utility in the adjuvant setting. Two clinical trials are currently ongoing for the adjuvant use of ipilimumab. The Eastern Cooperative Oncology Group (ECOG) 1609 clinical trial is currently underway has three treatment arms: high-dose ipilimumab, lowdose ipilimumab, and HD-IFN-a (see Figure 1).36 In Europe, the European Organisation for Research and Treatment of Cancer (EORTC) 18071 trial, comparing ipilimumab against placebo, has completed accrual and results are pending.37 Given the high cost and toxicity of ipilimumab, biomarkers of response would be useful to identify relevant patient cohorts. Elevated tumor-infiltrating lymphocyte (TIL), T reg, and indoleamine 2,3-dioxygenase (IDO) levels, have been associated with improved outcomes in patients receiving ipilimumab therapy in the first-line setting.38
Granulocyte-macrophage Colony-stimulating Factor
Granulocyte-macrophage colony-stimulating factor (GM-CSF) may be effective as an adjuvant therapy in stage III patients.39 A phase III trial in stage IIIB, IIIC, and IV disease, found that GM-CSF improved the RFS with minimal toxicity.40 A single-center study has also provided evidence for the efficacy of GM-CSF in this setting.41 There is a need for further clinical trials investigating its use and it may be that in combination with other immunotherapy we will see enhanced results.
A phase II trial found that the combination treatment regimen of GM-CSF and IL-2 in the adjuvant setting appeared to benefit high-risk melanoma patients.42 In a new approach to adjuvant therapy, a pilot study investigated the administration of a single short course of GM-CSF and IL-2 intradermally at the primary site, prior to its excision. This very small study underscores the biological activity of GM-CSF and IL-2 when given intradermally, and suggests that although newer studies are focusing on other agents, the addition of intradermal injections of IL-2 and GM-CSF to other adjuvant treatment schemes may prove beneficial.43
Melanoma vaccines have been extensively studied in the hope of obtaining durable clinical responses. Several large randomized trials of adjuvant allogeneic melanoma cell-based vaccines have been conducted, none of which have found any survival benefit.44–46 A polyvalent vaccine, Canvaxin, was evaluated as adjuvant therapy in stage III melanoma patients in a retrospective study. Median and 5-year OS were higher in vaccinated patients than in nonvaccinated patients.47 However, in a subsequent phase III trial for resected stage III/IV melanoma, Canvaxin did not increase either RFS or OS. In fact, survival was lower in the vaccine group (5% in stage IV and 9% in stage III) possibly a result of vaccine-induced immunosuppression.48 A recent phase III study, which randomized patients with stage II melanoma to adjuvant ganglioside GM2-KLH/QS-21 vaccination (n=657) or observation (n=657), found that this vaccine did not improve outcomes.49
Phase I/II trials have demonstrated an immunologic response from postoperative vaccine with melanoma -specific antigen A3 (MAGE-A3) in stage IV melanoma.50 The MAGE-A3 Antigen-Specific Cancer Immunotherapeutic (ASCI) combines a tumor-specific antigen delivered as a recombinant protein with an immunostimulant AS15. Unfortunately, a phase III trial investigating the use of MAGE-A3 ASCI as an adjuvant treatment for resected stage III MAGE-A3 positive melanoma patients has completed accrual and preliminary results suggest no benefit as reported by GlaxoSmithKline.51
Preliminary results of a trial comparing dendritic cell (DC) vaccine therapy with observation in stage III and IV melanoma patients (n=108) postsurgery presented at ASCO 2012 showed that immunotherapy with DC vaccine is safe and improves RFS compared with observation in the adjuvant treatment of stage III and IV melanoma. At a median follow up of 22 months, the HR for RFS for DC vaccine versus observation was 0.45 (95% CI 0.29–0.69; p<0.05) and for OS was 0.71 (95% CI 0.40–1.25; p=0.23).52 A peer-reviewed publication of this dataset is eagerly anticipated.
Radiotherapy
Radiotherapy may be valuable in the adjuvant setting in patients with bulky disease of involvement of nodes, particularly for those with the potential for a highly symptomatic nodal relapse.6 The use of radiotherapy as adjuvant has been investigated in a phase III trial, but although it improved regional control, it did not improve OS.53 Further work from this group presented at this last year’s ASCO confirmed the RFS advantage, but lack of OS and trend toward decreased quality of life suggest careful selection of patients where decrease in relapse outweighs the potential morbidity in terms of lymphedema risk.54
Targeted Agents
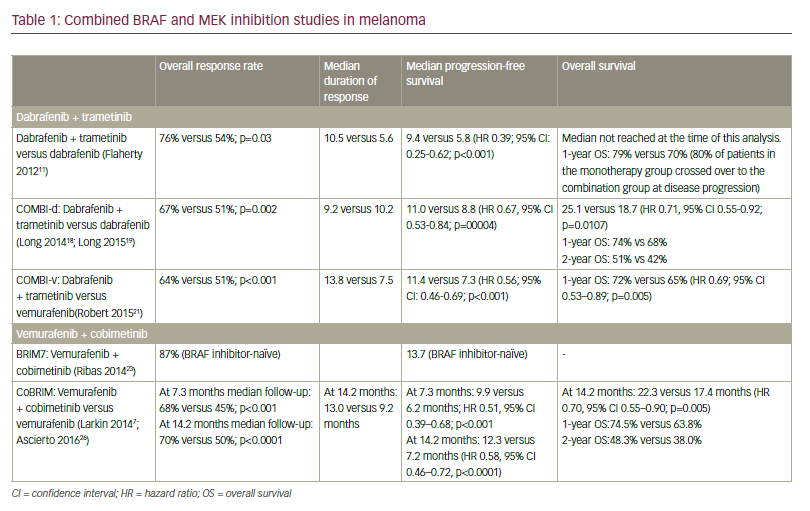
Approximately 40 to 60% of advanced melanomas have mutations in BRAF, and the BRAF inhibitor vemurafenib has demonstrated efficacy in the first-line setting,55 which has led to the suggestion that this agent may be useful as adjuvant therapy in stage IV disease. A phase III study of vemurafenib (RO5185426) adjuvant treatment of patients with surgically resected, cutaneous BRAF mutant melanoma at high risk for recurrence is currently ongoing.56 Further work has demonstrated improved response rates and PFS with combinations of b-raf and mek inhibitors.57,58 A second study comparing dabrafenib and tremetenib together versus observation is also underway.59
Prognostic Biomarkers and Predictive Factors for Response to Adjuvant Therapy in Melanoma
There is a need for prognostic biomarkers of IFN-a response, since identification of the ~15% of patients who will derive benefit from IFN-a would increase the therapeutic index of this agent. Numerous candidate molecules have been studied, including methylthioadenosine phosphorylase expression, YKL-40, S100B, melanoma-inhibiting activity, and tumor-associated antigen 90 immune complex.6 However, there is a lack of prospective data validating the use of these biomarkers. Serum protein S100B has been demonstrated to significantly correlate with mortality risk when assessed at baseline in patients with high-risk resectable melanoma.60
IFN-a-2b upregulates STAT1, a molecular molecule of progression, and downregulates STAT3 in tumor cells and host lymphocytes, The pSTAT1/ pSTAT3 ratio in tumor cells at baseline may serve as a useful predictor of clinical outcome in melanoma; the modulation of this ratio may serve as a predictor of therapeutic effect.61
Detection of circulating tumor cells (CTC) by molecular approaches may also be a promising prognostic biomarker in melanoma patients. In a recent phase III clinical trial, blood levels of three messenger RNA (mRNA) biomarkers of CTCs (MART-1, MAGE-A3, and PAX-3) status were significantly associated with RFS (HR 1.64; p=0.002) and OS (HR 1.53; p=0.028) before and during adjuvant treatment for resected stage IV melanoma patients.62
Lower tumor stage and ulceration have been noted as predictive factors for patient response to PEG-IFN-a-2b.27 Analysis of the results of the PEGIFN- a trial found that ulceration of the primary tumour was not only a strong prognostic factor, but also a significant predictive factor for patient response to adjuvant IFN-a treatment.27 There is also evidence that patients treated with IFN-a may benefit more if they have micrometastases than if they have macrometastases of the lymph nodes.63 In addition, the appearance of autoantibodies or clinical manifestations of autoimmunity during treatment with IFN-a-2b was also associated with improvements in RFS and OS in patients with melanoma.64 This observation was confirmed in a subsequent study65 and warrants further investigation. A phase III study of PEG-IFN-a-2b in high-risk, stage II patients with ulcerated melanomas (EORTC 18081) is ongoing.66
Summary and Concluding Remarks
Numerous clinical data support the efficacy of adjuvant IFN-alpha in the treatment of melanoma – although it is associated with consistent improvements in RFS, its effect on OS has been small. Future research should focus on determining the scientific basis of action of IFN-a, the optimum duration of therapy, as well as how to overcome resistance to further enhance efficacy of IFN-a. In particular, there is a need for clinical trial data in the highest risk category: resected stage IIIb-IV disease. An increased understanding of biological determinants of response, such as the induction of autoantibodies, as well as the identification of the biomarkers of subpopulations of patients who are likely to benefit the most from IFN-a therapy, will increase the effectiveness of clinical trials and provide further insights into the mechanisms of IFN antitumor activity.
The lack of treatments with proven efficacy against resected melanoma has affected the investigation of adjuvant treatment in melanoma therapy. Chemotherapy, cytokines, vaccines, and combinations of these treatments have been investigated, but with little success to date. Ongoing melanoma adjuvant trials are now testing the next generation of immunotherapy, as well as targeted agents. The complete and durable responses observed in phase III trials of ipilimumab in patients with metastatic melanoma suggest that this agent may demonstrate efficacy in the adjuvant setting. Although targeted agents, such as vemurafenib and combination treatment with dabrafenib and trametenib, may be beneficial and less toxic than HD IFN-a, their effects in stage IV disease appear to require continuous administration. In the adjuvant setting it may be that given their high response rate, micrometastatic disease will be eliminated at a similarly high rate and, if so, long-term benefits would be possible without continued treatment.
Although programmed cell death 1 (PD-1) blockade appears to be an interesting new immunotherapeutic its ability to produce treatment-free complete responses remains unknown. The favorable toxicity profile of the PD-1 inhibitors, in addition to the highest response rates yet documented for single-agent immunotherapeutics in treating stage IV patients, make them attractive agents for clinical study in the adjuvant setting. Future work in the adjuvant setting will be further informed by the efficacy of single agents, and combinations in metastatic disease.72,73