While the melanoma etiologic pathways are critical in developing prevention strategies, the increase in this disease has propelled melanoma into the sixth most common cancer and a significant cause for death of patients in their prime of life. Metastatic disease remains a formidable hurdle for oncologists today.
The prognosis of a patient with melanoma is primarily related to the depth of invasion into the skin as measured by Breslow thickness (T-stage), whether regional nodal spread is present (N-stage), and whether distant metastases have developed (M-stage).Within the group of patients with distant disease, the prognosis is determined by the site and burden of metastases.Patients with distant skin, subcutaneous or nodal metastases only (M1a) have a median survival of 12.8 months; patients with lung metastases (M1b) have an 11.8-month median survival; and patients with visceral metastases or elevation of lactate dehydrogenase (LDH) (M1c) have a 7.8-month median survival. Metastatic melanoma arising from the choriod of the eye (the second most common site for melanomas to develop) has an even poorer response to treatment than metastases arising from primary skin location, suggesting that choriodal melanomas have a different clinical biology and should be considered a separate disease.
Simple wide excision of the primary site is curative when cutaneous melanomas are found early and have a shallow depth of invasion. Unfortunately, once distant metastases have developed,therapeutic options are limited and those available have had little impact on survival except in a small minority of patients.
Those treatments that do provide durable remissions in a small minority of patients are typically associated with a high likelihood of serious adverse events including a risk of death. Therefore, an open discussion of expectations, risk, and benefits with the patient and their family is an important first step in setting the stage for intervention.
As with other types of cancer that have limited treatment possibilities, participation in clinical trials designed to evaluate new therapeutic strategies should be considered as a first-line option. Many new treatment strategies being evaluated in phase II and randomized phase III studies are available through national cooperative groups or through the National Cancer Institute’s Clinical Trials Program and may be accessible in the community oncologist office. If not, referral to a melanoma center should be considered.
Current standard therapy for metastatic melanoma includes chemotherapy, biotherapy, and bio- chemotherapy. These treatments have never been tested against best supportive care, making it difficult to form firm conclusions about clinical benefit. Since the 1970s, numerous phase II studies have identified several alkylating agents with activity, most notably dacarbazine (DTIC) and carmustine (BCNU). DTIC has an objective response rate of 19%, with a median duration of response of four months. The six-year survival for metastatic melanoma patients treated with DTIC is less than 2%.Temozolomide (TMZ), an oral alkylating agent, is chemically converted in the body to monomethyl triazenoimidazole carboxamide (MTIC), the active metabolite in DTIC. By virtue of being oral, TMZ allows exploration of low dose chronic administration. One advantage of this approach is the ability to modulate O (6)- methylguanine-DNA methyltransferase, one of the major resistant pathways for this class of alkylating agents. Of note is the observation that TMZ induces significant changes in peripheral blood helper T-cell status, resulting in an increased risk of opportunistic infections. The exploratory studies with TMZ have not yet been confirmed in larger multicenter trials. In a randomized study comparing fotemustine, a third generation nitrosurea, and DTIC, fotemustine was associated with a slightly higher response rate compared with DTIC (15% versus 7.2%) and a 1.6- month survival advantage.
Over the past 30 years, numerous combination chemotherapy regimens have been used, but ultimately failed to stand up in randomized studies. Three combination chemotherapy regimens are in use throughout the US today: CDDP/VBL/DTIC (CVD); DTIC/BCNU/CDDP/Tamoxifen (Dartmouth Regimen); and thalidomide and temozolamide (TT). In phase II studies, these regimens have induced objective response rates of 20% to 50%, with single institutions reporting long-term remissions, particularly in those patients achieving complete remission. In a randomized study comparing the Dartmouth Regimen with single- agent DTIC, the response rate for the combination therapy was 18.5% versus 10.2% for DTIC alone (p=0.09); however, there was no survival advantage.
Generally, response rates associated with multi-agent chemotherapy have been slightly higher than those of single agents, but no multi-agent chemotherapy has been associated with survival advantage. Thus, combination chemotherapy is considered for selected patients in whom the reduction of tumor burden would palliate significant cancer-related symptoms.
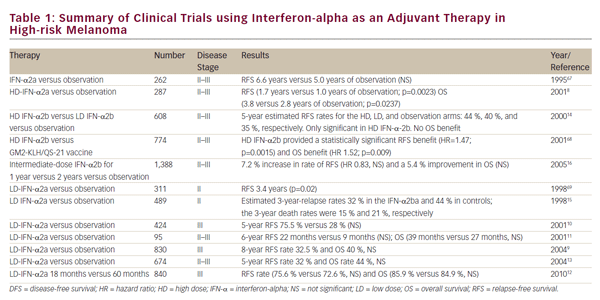
In the mid 1980s, interferon (IFN) and interleukin (IL)-2 showed modest single-agent activity, with long survival of a few patients. Interferon alpha (IFN-_) has some modest activity in stage IV disease and is used primarily as adjuvant therapy in surgically resected high-risk stage II and III disease.While response rates to biotherapy with high-dose bolus IL-2 have been low (16% objective response rate; 8% complete response rate), approximately 60% of patients who enter a complete remission survive for more than 10 years.This minority of patients with significant clinical benefit is the rationale for the US Food and Drug Administration’s (FDA) approval of IL-2 a decade ago.
High-dose bolus IL-2 has significant toxicity and requires hospitalization in a unit capable of providing cardiovascular monitoring and blood pressure support, which is commonly needed. Because there is significant skill required to administer high-dose bolus IL-2, competency is related to volume of patients treated. For these resaons, high-dose bolus IL-2 is typically offered only at a limited number of centers. Clinicians who are intent on providing this therapy in the community hospital setting would best plan carefully and consider making a site visit with their team of healthcare providers to one of the centers that employs high-dose IL-2 therapy. For patients who are willing and can tolerate this therapy, high-dose bolus IL-2 is considered first-line therapy because of the long-term survival benefit seen in approximately 8% of patients. For theoretical and clinical reasons, combining chemotherapy and biotherapy is an attractive concept.
The first studies of IFN and DTIC looked initially promising. A follow-up large multi-institutional randomized study of IFN+DTIC versus DTIC or IFN alone failed to confirm an advantage of combination over single agent DTIC. Combining chemotherapy with IL-2 and IFN is commonly referred to as bio- chemotherapy and has been reported to induce high response rates and long-term survival. Combined modality therapies that employ a high-dose IL-2 backbone are given in hospital where the necessary services to support these individuals are available.
Recent enthusiasm for bio-chemotherapy has been based on success reported from single institution, single arm trials and precipitated a number of larger single institution and multi-center randomized studies comparing different variations of bio-chemotherapy with combination chemotherapy.Two studies (NCI and the Italian Group) failed to find any statistically significant improvement in response rates or survival.
The third study (MD Anderson) demonstrated a doubling of objective response rates (48 versus 25%; p=0.001) and of time to progression of disease (4.9 versus 2.4 months; p=0.008), but no advantage for overall median survival (18.7 versus 15.4 months; p=0.99).These findings generated renewed enthusiasm for biochemotherapy. Subsequent studies have now been completed. The US intergroup study compared IL-2/IFN+CVD with CVD, in 397 patients. Primary end-points included response, survival, and time to progression (TTP). The median overall survival (OS) was 8.1 for biochemotherapy compared with 8.7 months for chemotherapy alone (p=0.439); TTP was about four months for biochemotherapy and approximately two months for chemotherapy (p=0.082). Significantly more toxicity was noted in the biochemotherapy arm.
A meta-analysis suggests that biochemotherapy may offer a small advantage over chemotherapy or IL-2 alone, which leads us to conclude that there may be a small subset of patients who benefit from biochemotherapy.At this time,the data for biochemotherapy is not compelling for its adoption as standard therapy.
Newer strategies of therapy have begun to take shape as understanding of melanoma biology and immunology has grown. Agents that target specific tumor cells and vascular growth pathways are available and have entered clinical trials. This better understanding of immune regulation and mechanisms by which melanoma can evade immune destruction has lead to new therapeutic immune strategies being tested in clinical trials. It is with great hope and expectations that these new agents and strategies will have a major impact on the treatment of metastatic melanoma.
To date, there are no markers that aid an oncologist in determining which patients will or will not respond to any of the treatments available. It is hoped that the advent of molecular and protein profiling of patients and their tumors will provide a new era in selection of therapy for metastatic melanoma patients.The management of patients with metastatic melanoma is entering a new era of exploration in the hope of identifying better therapies.Nevertheless,the practitioner is still faced with making choices for care today. One should first consider participation in a clinical trial for appropriate patients. When there are limited sites of metastases and the clinical situation permits, a surgical approach (metastatectomy) is offered. This approach is based on case series that suggest a subset of patients who undergo metastatectomy have a prolonged survival. For younger patients with good cardiopulmonary, renal, and liver function, single agent high-dose IL-2 therapy is considered. For older patients, and those with slow- growing disease, single agent chemotherapy with either DTIC, FOT, or TMZ is reasonable. If there is rapid progression of disease and significant cancer-related symptoms,the use of combination chemotherapy or bio- chemotherapy is considered.
Once a first-line therapy has failed, secondary treatments can be considered.Again, participation in a clinical trial is reasonable including use of never-before- used-in-man agents being tested in phase I trials.
Paclitaxel alone or in combination with platinum analog has also been shown to provide some palliative benefit in this setting.
In conclusion, when considering the care of patients with metastatic melanoma, the clinician should carefully evaluate each patient’s individual needs and expectations, co-morbid conditions, treatment choices, treatment toxicity, and likelihood of clinical benefit. In the author’s experience, including the patient and their family in decision-making affords clear understanding of expectations and reality, providing an environment that is supportive and caring.