Despite progress in managing many cancer types in recent decades, metastatic pancreatic ductal adenocarcinoma (PDA), accounting for over 90% of all malignancies of the pancreas, continues to have an extremely poor prognosis. Indeed, in the US PDA has an estimated 5-year survival rate of 8% for all patients and 3% for metastatic disease.1,2 PDA is now the third leading cause of cancer death in the US and this is predicted to increase to be the second biggest cause of cancer death in the US by 2020–2030.3,4
Outcomes in PDA have not improved substantially over the last 30 years.1 This mainly arises from the patient having reached a metastatic stage at the time of diagnosis with poor response to therapy. The lack of progress in developing effective therapies for PDA is due to the nature of the disease: tumor cells co-opt multiple cellular and extracellular mechanisms to form a complex cancer organ with a marked propensity towards metastasis and resistance to therapy.5 Against this bleak picture, improved understanding of the pathophysiology behind PDA, the identification of molecular mechanisms underlying its aggressive nature, and improved access to the tumor site have enabled some exciting developments. This increased knowledge raises hope for more successful approaches to managing this disease.
The tumor microenvironment
The development, progression and aggressiveness of PDA are significantly affected by components of the tumor microenvironment (TME), which include a dense extracellular matrix (ECM), fibroblasts, inflammatory cells, and abnormal vasculature.6 The TME can profoundly influence tumor behavior and response to therapy, and as a result it has emerged as an important target for therapy and as a major focus of cancer research.7 Within the TME, hyaluronan (HA) is a naturally occurring linear polysaccharide, and a major component of the tumor stroma in many solid tumors, including PDA.8 In animal models, accumulation of HA increases tumor interstitial fluid pressure (IFP), which in turn compresses blood vessels and compromises blood flow.9–12 This process leads to hypoxia in the tumor, which is a contributory factor in metastasis. In response to low oxygen levels, tumor cells trigger the synthesis of hypoxia-inducible factor 1a (HIF1a), a protein that directly represses the transcription of miR-34a microRNA. Furthermore, this down-regulation of the microRNA is a prerequisite for hypoxia-induced epithelial-to-mesenchymal transition (EMT). In this process, HIF1a activates a genetic program that results in the transformation of non-invasive cells (which grow in a regulated fashion in epithelial sheets) into invasive, migratory cells that can develop new tumors elsewhere.13,14
Accumulation of HA in tumors may also act as a barrier to immune cells, including T lymphocytes and macrophages, creating an immune suppressed microenvironment.15–17 Increased HA also prevents chemotherapeutic agents and monoclonal antibodies from reaching their sites of action.8,9 HA accumulation in the TME is associated with accelerated tumor growth and is an independent, negative predictor of survival.18–24
PEGylated recombinant human hyaluronidase
Mechanism of action
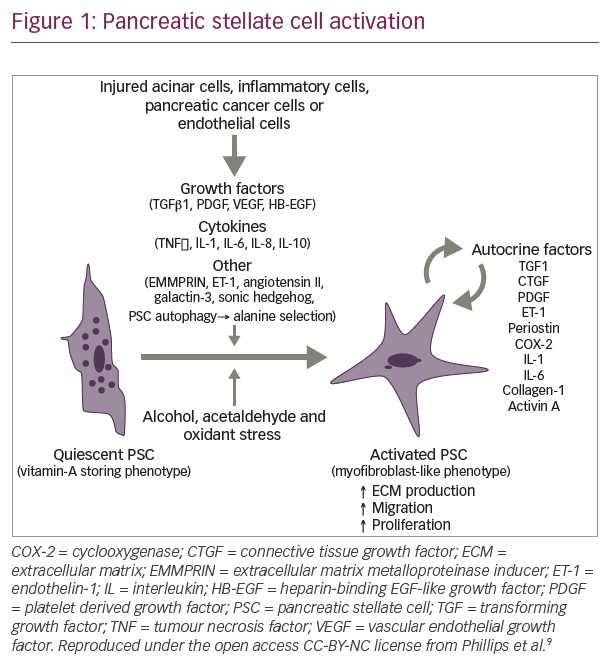
A notable development in targeting HA in PDA has been the emergence of the novel investigational agent, PEGPH20 (pegvorhyaluronidase alfa), which is the PEGylated form of the recombinant human hyaluronidase, PH20. PEGylation of this enzyme prolongs circulatory time to facilitate HA degradation in the extracellular matrix surrounding tumor cells, enhancing consequent remodeling of the tumor stroma (see Figure 1).12
In animal models and in studies on various types of cancer, accumulation of HA has been shown to promote tumorigenesis.8,9,12,25,26 Conversely, HA depletion reverses these changes and inhibits tumor growth. However, the precise mechanism of PEGPH20-induced HA depletion is not completely understood.27 Preclinical studies show that treatment with PEGPH20 increases vascular perfusion in the tumor, which decreases or eliminates hypoxia and causes other alterations in the TME.8,12 Other in vivo studies in both animal and human tumors show that PEGPH20 decreases hypoxia-related protein expression and induces translocation of proteins such as E-cadherin and β-catenin to the plasma membrane.8
Preclinical studies have also shown that PEGPH20-mediated HA reduction facilitates immune cell access to the TME, and increases CD8+ T cell recruitment to tumor sites.17,28 In animal models of PDA treated with PEGPH20 plus chemotherapy or monoclonal antibodies, the biophysical effects of HA degradation by PEGPH20 translate into reduced tumor growth and improved survival.9,10,12,29,30 In addition, adenosine, antibodies, and other molecular receptors are involved with dampening inflammation caused by HA degradation.
In patients with PDA and in other malignancies, levels of HA in tumors are variable, but tumors that accumulate HA (termed HA-High tumors) are more likely to respond to PEGPH20 treatment. In such tumors this treatment mediates HA reduction and this has been shown to decrease tumor hypoxia and consequently reduce metastases.31,32 In vitro and in vivo studies have shown that depletion of HA as a result of treatment with PEGPH20 leads to increased natural killer (NK) cell access to HA-High tumor cells and enhanced trastuzumab or cetuximab-antibody-dependent cell-mediated cytotoxicity.17 This further supports the belief that surrounding PDA tumors, the HA matrix forms a barrier that inhibits monoclonal antibody and NK cell access; this barrier can be overcome using PEGPH20. In tumor cell membranes, CD44 receptors and receptors for hyaluronan mediated motility (RHAMM) bind and anchor the HA matrix, and this association is believed to enhance receptor tyrosine kinase activity that drives tumor progression and increases treatment resistance.27 Therefore, PEGPH20 may have some antitumor properties resulting from reduced signaling via CD44/RHAMM.
Overall, the preclinical and clinical evidence indicates that PEGPH20 has multiple antitumor effects but all are believed to result in substantial changes in the TME. These effects may inhibit progression, reduce tumor interstitial fluid pressure and improve vascular perfusion in HA-High tumors, resulting in increased access by immune cells, antibodies and anticancer therapies.9,10,12,29
Clinical studies
Early studies of single-agent PEGPH20 in patients with metastatic cancer, including PDA, identified muscle spasms, arthralgia, and myalgia as dose-limiting; maximum tolerated dose was found to be 3 µg/kg twice weekly.33 In a subsequent phase Ib trial of PEGPH20 plus standard-dose gemcitabine in patients with previously untreated metastatic PDA, a recommended phase II dose for PEGPH20 (3 µg/kg) was confirmed.34 Adverse events were again primarily musculoskeletal but did not lead to discontinuation. Exploratory imaging analyses in a subset of patients showed an increase in tumor perfusion at 24 hours, as assessed by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), and a sustained reduction in tumor metabolic activity by fluorodeoxyglucose positron emission tomography with integrated computed tomography (F-FDG-PET/CT); these findings support the described mechanism of action of PEGPH20 that HA degradation improves access to the tumor site, thereby increasing exposure of systemically administered anticancer therapies. This study also demonstrated that patients with HA-High tumors might benefit most from PEGPH20-based treatment, a premise studied in a phase II trial and in an ongoing randomized, double-blind phase III trial.
More recent clinical findings that support PEGPH20 use in PDA come from a phase II, randomized, multicenter study in patients with stage IV, previously untreated disease (the HALO 109-202 study, NCT01839487; n=279).34 Patients received PEGPH20 plus nab-paclitaxel and gemcitabine (PAG), or nab-paclitaxel and gemcitabine (AG) chemotherapy alone. In this study, HA was tested as a biomarker to potentially identify patients who may be more likely to benefit from PEGPH20 treatment. Tumor samples – formalin-fixed paraffin-embedded tissues from core samples – were analyzed using an affinity histochemical assay (VENTANA HA RxDx Assay), co-developed by Halozyme Therapeutics, Inc. and Ventana Medical Systems Inc. (Tucson, AZ, USA), to analyze levels of HA in solid tumors and potentially predict which patients might better respond to the addition of PEGPH20 in cancer treatments.35 This assay has been reported to have an inter-reader precision level of 94%.35
The HALO 109-202 study was conducted in two stages. Stage 1 data (n=146) comprised the 'training set' for development of the VENTANA HA RxDx Assay and scoring algorithm.36 Patients with untreated metastatic PDA were randomized 1:1 to PAG twice weekly plus AG weekly, or AG once every 28 days. In this stage, approximately 40% of patients in the PAG arm during stage I of the trial developed a thromboembolism (TE), and as such were removed from study participation. The trial was consequently put on hold while the protocol was amended to include TE prophylaxis. TEs are a well-established complication of metastatic PDA and are further compounded by chemotherapy administration. As a result of this experience, in stage 2 patients at high risk of TE were excluded from the study and prophylactic enoxaparin was initiated. Under the revised protocol, stage 2 enrolled an additional 133 patients who were randomized 2:1 to PAG or AG treatment. The proportion of patients with HA-High tumors was 32% in stage 1 and 28% in stage 2.
Data from stage 1 were used to select the 50% cut-off point for HA (i.e. the 50% HA level from the range of HA levels found in study patients' tumors).37 In this respect this study was novel in identifying a biomarker with the potential to guide patient selection. Combined data from stages 1 and 2 of the HALO 109-202 study presented at the Annual Meeting of the American Society for Clinical Oncology, 2017,38 show a robust signal for improved progression-free survival (PFS) when the cut-off for classifying patients as HA-High using the Ventana HA RxDx assay was set at ≥50%. Using this criterion, of 246 patients with HA data, 84 were classified as HA-High. The primary endpoint of PFS was significantly higher for PAG versus AG treatment: 6.0 months versus 5.3 months, respectively; hazard ratio (HR) 0.73; 95% confidence interval (CI) 0.53–1.00; p=0.048. A PFS increase of 4 months in pancreatic cancer is a clinically relevant and substantial improvement. This result was even more striking in HA-High patients (9.2 months versus 5.2 months, respectively; HR 0.51; 95% CI 0.26–1.00; p=0.048).38
In the combined HALO 109-202 study analysis, TEs were primarily venous in nature but incidence was comparable between treatment arms (14% for PAG versus 10% for AG) following enoxaparin initiation. Treatment-related adverse events of any grade associated with either PAG or AG treatment included peripheral edema (63% for PAG versus 26% for AG), muscle spasms (56% versus 3%), neutropenia (34% versus 19%), and myalgia (26% versus 7%).38
The HALO 109-202 study therefore met its key endpoints including improvement in the primary outcome of improved PFS in all patients receiving PAG compared with AG, and in the secondary endpoint of improved PFS in HA-High patients receiving PAG versus AG. Stage 2 of the HALO 109-202 study also met its primary safety endpoint of TE reduction. Positive trends in PFS and overall survival (OS) were observed in HA-High patients, supporting the ongoing phase III investigation of PAG versus AG in patients with HA-High PDA.38
To date, there have been no studies investigating PEGPH20 as monotherapy in PDA. All studies have investigated PEGPH20 used in combination with chemotherapy. PEGPH20 is currently under investigation in two small studies: one is investigating PEGPH20 for the treatment of patients with PDA without metastasis prior to resection surgery (NCT02241187), and one is investigating the treatment of patients with unresectable PDA (NCT02910882). Favorable findings in these trails and larger trials could increase indications in PDA suitable for PEGPH20 treatment.
Perspective and future directions
Pancreatic cancer is characterized by a profuse desmoplastic stroma, which is associated with aggressive pathogenesis and treatment resistance.6,39 This HA-rich stroma compresses tumor vasculature and provides a barrier to delivery of antineoplastic agents, including chemotherapeutics and therapetic antibodies, and immune cell infiltration. Overcoming this barrier offers an innovative strategy for improving anticancer therapy that may be applicable to multiple diseases at varying stages.
Previous efforts to target the PDA TME, however, have not been as successful. Specifically, clinical trials evaluating the roles of vascular endothelial growth factor inhibitors and the Hedgehog pathway inhibitor, IPI-926, have yielded disappointing results.40 Other approaches to potentially address the TME in PDA include treatment with heparin sulfate mimetic, M402 (NCT01621243), secreted protein rich in cysteine (SPARC)41 or evofosfamide;42 however, as yet, these approaches have not shown any notable evidence of efficacy. Targeting HA is a novel approach to address the vital role the microenvironment plays in PDA pathogenesis.
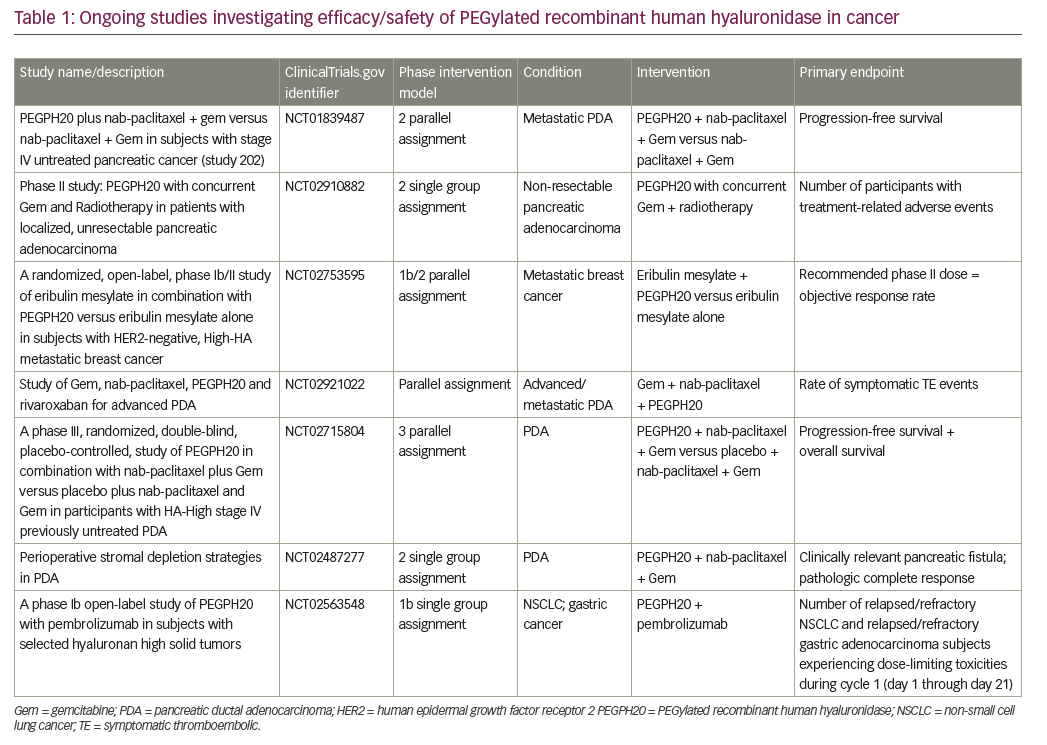
Currently, many therapies targeting PDA, TME and HA are under investigation (Table 1).43–46 HA degradation by PEGPH20 is the most studied concept to date and is the most advanced in terms of development. Multiple preclinical and clinical studies have demonstrated that targeting the biophysical barrier to drug delivery in PDA with PEGPH20 is both feasible and promising.12,25,34,37 Results from both the phase Ib34 and phase II (HALO-202)38 clinical trials have demonstrated that the administration of PEGPH20 with different cytotoxic chemotherapy combinations is feasible and effective.
The results from the phase II HALO 109-202 study, in particular, demonstrated that the ≥50% HA cut-off limit can be used as a reliable biomarker to screen patients who may benefit from PEGPH20 treatment.38 This was a novel aspect of the study and showed that the level of HA in patients receiving PEGPH20 is a critical factor, and is one of the only predictive biomarkers currently under prospective evaluation in PDA. The HALO 109-202 study also demonstrated improved efficacy of PEGPH20 in combination with AG compared with AG alone in terms of PFS and secondary endpoints (PFS by HA level, overall response rate and overall survival), especially for HA-High patients. These exciting findings justify and support the ongoing phase III HALO 109-301 study (NCT02715804), in which a larger patient population (n=420), all of whom have HA-High tumors (using the same ≥50% HA cut-off point), are randomized to treatment with PAG or AG. This pivotal study incorporates PFS and OS as co-primary endpoints and will provide key data on the utility of PEGPH20 in metastatic PDA treatment.
The risk of TE that emerged in the HALO 109-202 study has been shown to be mitigated when PEGPH20 is given concurrently with prophylactic low molecular weight heparin anticoagulation therapy. Additional toxicities attributed to PEGPH20 include edema, neutropenia, and musculoskeletal effects (MSKEs; myalgia and muscle spasms). The musculoskeletal effects associated with this medication are quite unique. Patient-reported muscle cramping is an uncommon drug-related toxicity with few standard treatment approaches. MSKEs associated with PEGPH20 have been found to be quite manageable and are infrequently dose limiting or a reason for drug discontinuation. Anti-inflammatory medications, as well as cramping emollients, are both quite helpful, and MSKEs are reduced with use of corticosteroids (dexamethasone). In addition, patients may benefit from concurrent muscle relaxant, non-steroidal anti-inflammatory drugs (NSAID), or topical agents, as needed. In rare cases, temporary courses of opiate pain medications are needed for adequate pain control.
Overcoming therapeutic resistance through stroma-targeting agents, like PEGPH20, may provide an ideal complement to multiagent chemotherapy. Already, PEGPH20 is being studied in combination with one of the current standards of care for first-line chemotherapy, which is currently used to treat metastatic PDA: nab-paclitaxel/gemcitabine. However, recruitment to a SWOG (formerly Southwest Oncology Group) phase Ib/II trial investigating the addition of PEGPH20 to FOLFIRINOX in patients with untreated PDA has been permanently closed to enrolment due to a predicted, unlikely statistically significant, advantage in OS.47 Despite this setback, we envision a future where PEGPH20 may be combined with, and improve efficacy of, other antineoplastic regimens to treat both early-stage and metastatic PDA.
The development of PEGPH20 as a stromal targeting agent, and the identification of a subgroup of patients more likely to benefit from it, is changing the treatment paradigm of PDA. The VENTANA HA RxDX Assay for selection of patients with HA-High tumors is one of the only potentially predictive biomarkers for PDA under prospective evaluation. This biomarker will allow us to stratify PDA patients into groups most likely to benefit from PEGPH20-mediated HA degradation and allow HA-low patients to pursue alternative approaches. HA-degradation by PEGPH20 is also being studied in other tumor types, including breast, gastric and non-small cell lung cancers (NSCLC), and will soon be studied in combination regimens for other HA-High cancers.
Preclinical studies have also demonstrated that the malignant stroma that is rich in HA restricts tumor immune cell and antibody infiltration. The combination of PEGPH20 and pembrolizumab, a humanized monoclonal antibody that targets programmed cell death receptor 1 (PD1), is currently being studied in patients with cancers such as NSCLC and gastric cancers (NCT02563548). Overcoming the stromal barrier may extend the benefits of PD1 blockade to populations whose disease has been refractory to single agent checkpoint inhibitors, as we have seen in PDA and other diseases.
Advances in the understanding of the PDA TME and the development of agents targeting HA are rapidly changing the PDA treatment landscape. Targeting the stroma, overcoming treatment resistance, and stratification of patients are providing a new framework for more successful treatment of this disease. This is allowing the barrier to effective PDA treatments to be broken, and provides a shift away from predominantly poor prognosis that oncologists have confronted hitherto. These new approaches have the potential to provide new hope and direction for the pancreatic cancer community.