Ovarian cancer accounts for 4% of all cancers in women and is the leading cause of death from gynaecological malignancies. In Europe, estimates suggest that 61,000 new cases of ovarian cancer are diagnosed and that the disease is responsible for 39,000 deaths each year.1 Epithelial ovarian carcinoma is usually diagnosed at an advanced stage. Only 30% of cases present with early-stage disease, defined as disease confined to the pelvis without spread outside the gynaecological organs (International Federation of Gynaecology and Obstetrics [FIGO] stages I and II). Although surgical excision offers definitive cure in a significant percentage of these patients, 10–50% eventually relapse, indicating that even early-stage disease represents a heterogeneous entity and emphasising the need to identify patients most likely to benefit from adjuvant treatment.2
The standard surgical procedure in early ovarian cancer consists of total hysterectomy, bilateral salpingo-oophorectomy, omentectomy and peritoneal lavage. Extensive staging by an experienced gynaecological oncologist performing a meticulous exploration of the entire abdomen, including the pelvic and the para-aortic lymph nodes, results in an upstaging in approximately one-third of patients.3 Based on these findings, the European Organisation for Research and Treatment of Cancer– Gynaecological Oncology Group (EORTC-GOG) defined an optimal staging pocedure for early ovarian cancer as: inspection and palpation of all peritoneal surfaces; biopsies of any suspected lesions; peritoneal washings; blind biopsies of the right diaphragm and right and left para-colic gutter, pelvic side-walls of the ovarian fossa and the bladder peritoneum; and sampling of iliac and para-aortic lymph nodes.3 Complete surgical staging in early-stage ovarian carcinoma is of paramount importance for optimal management, as misclassification can lead to suboptimal treatment.4
Prognostic Factors
A number of studies have proposed clinical and pathological variables, such as age and FIGO stage at diagnosis, degree of differentiation, histological type, extracapsular invasion or large-volume ascites (FIGO stage IC) and rupture during surgery as important prognostic factors for early-stage epithelial ovarian cancer in multivariate analysis.5–14 Among these, degree of differentiation has been the only factor consistently possessing independent prognostic value in all published multivariate analysis. Therefore, grade of differentiation is an important prognostic factor and should be used in the classification of risk and for therapeutic planning.1 Additional parameters, such as bilateral occurrence (FIGO stage IB), clear cell histology and positive peritoneal cytology, have been proposed as non-favourable prognostic factors in univariate analysis.5,9,10,14
Role of Optimal Surgical Staging
The fundamental role of optimal surgical staging in ovarian cancer has long been emphasised. In the Adjuvant Chemotherapy In Ovarian Neoplasm trial from EORTC (EORTC-ACTION trial), which compared adjuvant chemotherapy with observation in early-stage ovarian cancer, only onethird of the patients had adequate surgical staging procedures in both arms of the study.15 Among patients in the observation arm, optimal staging was associated with a substantial survival benefit. No such association was observed in the chemotherapy arm. In the non-optimally staged patients, adjuvant chemotherapy was associated with statistically significant improvement in recurrence-free and overall survival, whereas in the optimally staged patients no benefit of adjuvant chemotherapy was seen. On the other hand, in a parallel study16 conducted by the International Collaborative Ovarian Neoplasm 1 (ICON1) study group, thorough surgical staging with total hysterectomy and bilateral salpingooophorectomy was recommended as the minimum procedure. In that study, adjuvant chemotherapy was associated with improved survival. In the combined analysis of the two studies,17 a clear survival benefit was demonstrated for the experimental (chemotherapy) arm. Thus, it is very likely that the benefit from adjuvant chemotherapy may reflect only the ‘amount’ of unappreciated residual disease in non-optimally staged patients.4 Therefore, one could argue that optimally staged patients do not derive clinical benefit from adjuvant chemotherapy, whereas non-optimally staged patients do. Of note, among patients who were classified as stage I after optimal staging, relapse occurred in 10%, whereas in patients who were not adequately staged, had no risk factors and did not receive adjuvant chemotherapy, recurrence was seen in 28% (p=0.0036).5 In either case, the paramount role of optimal surgical staging in order to apply the most appropriate type of adjuvant chemotherapy should be emphasised.
Role of Adjuvant Chemotherapy
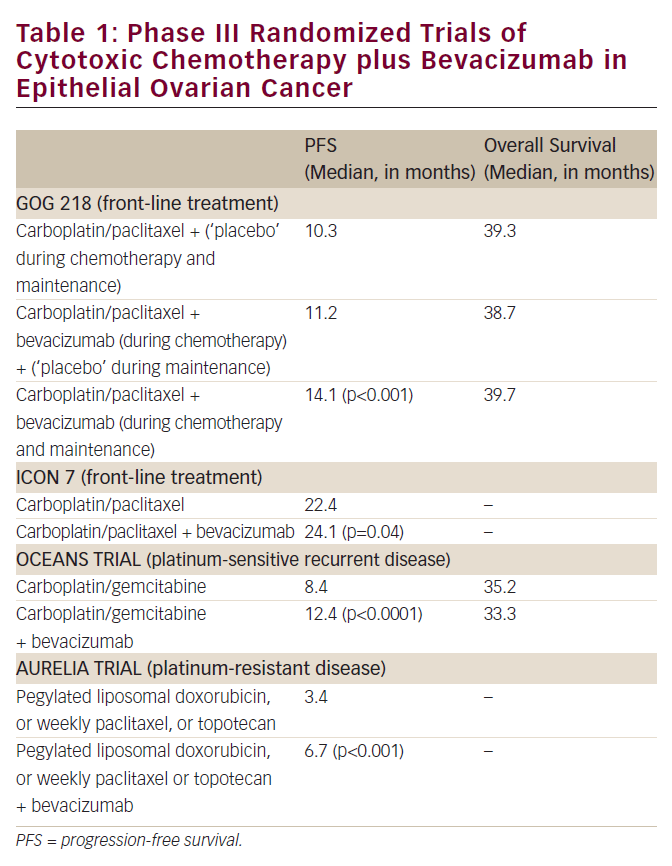
All published studies concerning adjuvant chemotherapy in early-stage epithelial ovarian cancer are presented in Table 1. Patients with FIGO stage IA and IB tumours of low histological degree (grade I) have demonstrated an excellent prognosis after adequate surgical staging (100% disease-free survival rate) and thus do not necessitate adjuvant treatment, with the exception of clear-cell histology tumours.18 For these patients, surgery alone is the treatment of choice. For the rest of earlystage ovarian cancer patients who present with high-risk factors, adjuvant platinum-based chemotherapy has been proposed as the best strategy to prevent recurrence compared with observation alone.
However, early trials conducted in the 1990s failed to demonstrate substantial survival benefit from adjuvant platinum-based chemotherapy in early-stage ovarian cancer, with the exception of a single study where cisplatin produced a significant improvement in disease-free survival.19 However, it should be noted that the above-mentioned trials lacked statistical power to detect realistic and clinically relevant differences. More recently, three large prospective European trials were conducted to clarify the role of adjuvant chemotherapy in poor-prognosis patients with early-stage epithelial ovarian carcinoma. The first study was conducted by the Nordic Co-operative Ovarian Cancer Group (NOCOVA) and showed no statistically significant benefit from adjuvant chemotherapy (six courses of carboplatin, AUC=7) compared with observation alone. However, the trial was closed prematurely due to poor accrual.11 In 2003, the results of two other large randomised clinical trials were published. In the abovementioned ICON115 and EORTC-ACTION16 trials, 925 patients (477 in the ICON1 trial and 448 in the ACTION trial) were randomly assigned to receive either platinum-based adjuvant chemotherapy or observation alone. In the ACTION study, eligible patients belonged to FIGO stages IA–IB grade II or III, FIGO stages IC–IIA (all grades) and clear-cell carcinomas independently of FIGO stage. After a median follow-up of 5.5 years, the difference in overall survival between the two trial arms was not statistically significant (HR 0.69, 95% confidence interval [CI] 0.44–1.08; p=0.10). However, recurrence-free survival was significantly improved in the adjuvant chemotherapy arm (HR 0.63, 95% CI 0.43–0.92; p=0.02).
In contrast, the ICON1 trial enrolled patients of all FIGO stages, the majority of them belonging to stages I and II.16 This study showed that women who received adjuvant chemotherapy had better overall survival than women who did not (HR 0.66, 95% CI 0.45–0.97; p=0.03). The combined analysis of the two trials17 demonstrated significant survival advantage in favour of the chemotherapy arm in terms of five-year overall survival (82 versus 74%; p=0.008) and five-year recurrence-free survival (76 versus 65%; p=0.001). Despite the methological arguments, these two studies were the first to provide solid evidence concerning the benefit of adjuvant chemotherapy in early-stage ovarian carcinoma.
Chemotherapy Regimen and Duration of Chemotherapy
As shown in Table 1, previous studies evaluated platinum-based regimens without taxanes, which were later included in the therapeutic armamentarium. Paclitaxel has recently emerged as an essential component of the first-line treatment for advanced ovarian cancer in combination with cisplatin or carboplatin after the results of four randomised studies.20–23
It has been suggested that patients with early-stage epithelial ovarian carcinoma should receive chemotherapy in the adjuvant setting and with the same regimen as is currently recommended for advancedstage disease (i.e. six cycles of carboplatin/paclitaxel), especially when important risk factors are present. The results of a GOG randomised phase III trial showed that, compared with three cycles, six cycles of carboplatin/paclitaxel combination do not significantly alter the recurrence rate in unselected women with early-stage epithelial ovarian cancer.24 A recent study from the Hellenic Co-operative Oncology Group (HECOG)25 evaluated the role of adjuvant carboplatin/paclitaxel chemotherapy in early-stage epithelial ovarian cancer on the basis of the Goldie hypothesis, which suggests that this active regimen should be more effective in early stages with less tumour burden. The authors concluded that, in patients with FIGO stage IA or IB grade II or III disease, as well as in patients with IC or II grade I disease, four cycles of the same regimen represent an active and safe alternative. Patients with FIGO stages IC or II and tumour grade II or III may benefit from a more extensive treatment, similar to the one given in the advanced stages (six cycles of carboplatin/palitaxel combination).25 Finally, in a more recent phase II trial,26 adjuvant chemotherapy with the carboplatin/paclitaxel combination in early-stage ovarian carcinoma yielded high response rates, with five-year recurrence-free survival as high as 79%.
Conclusions
All women with early-stage epithelial ovarian cancer should undergo complete surgical resection with optimal surgical staging. Patients with FIGO stage IA and IB tumours of low histological grade do not require adjuvant treatment in the absence of important risk factors (e.g. clear cell histology). The type of adjuvant chemotherapy as well as the optimal number of cycles for other patients remains debatable. As early-stage ovarian cancer represents a prognostically diverse malignant entity, further studies focusing on identification of the subgroups of patients most likely to benefit from adjuvant chemotherapy are warranted. ■
My Learning
Login
Sign Up FREE
Register Register
Login
Trending Topic

11 mins
Trending Topic
Developed by Touch
Mark CompleteCompleted
BookmarkBookmarked
Gabriel Lenz, Rafael Alvim Pereira, Milena Tumelero
NEW
Lung cancer is the second most common type of cancer worldwide and in the USA, with its incidence varying depending on geographic and socioeconomic factors.1–3 According to data from the Global Cancer Observatory: Cancer Incidence, Mortality and Prevalence (GLOBACAN) database, an estimated 2.48 million new cases of lung cancer were reported in 2022.4,5 Although lung cancer rates in the […]
touchREVIEWS in Oncology & Haematology. 2025;21(1):Online ahead of journal publication