Ovarian cancer is the most lethal gynaecological cancer. In the US, approximately 21,880 new cases were identified and 13,850 women died of the disease in 2010.1 In the European Union, the number of newly diagnosed cases was 42,700 in 2004 and the mortality rate is 12 per 100,000 women per year.2 The current treatment for ovarian cancer is aggressive cytoreductive surgery followed by platinum- and taxane-based chemotherapy, with an objective response rate of 65–80 %.3 However, 60–70 % of cancers recur and patients’ prognosis and quality of life remain poor.4
Platinum-based combinations are the standard treatment for patients whose cancer recurs more than six months after the initial therapy.5 Combination treatment with paclitaxel and gemcitabine has been associated with better outcomes than platinum monotherapy in platinum-sensitive patients with recurrent tumours.6 Recently, the CALYPSO trial – a multinational, randomised, Phase III study comparing pegylated liposomal doxorubicin (PLD) and carboplatin versus paclitaxel and carboplatin in patients with relapsed, platinum-sensitive ovarian cancer – reported a greater therapeutic index for the PLD plus carboplatin combination, which thus represents, for most clinicians, the preferred option in this setting.7
The response to platinum retreatment is related to the duration of the prior response to platinum.8,9 It has been postulated that patients with partially platinum-sensitive recurrent ovarian cancer (i.e., those with a platinum-free interval of six to 12 months before recurrence) may benefit from a strategy of artificially extending the platinum-free interval by using a non-platinum agent at first relapse, followed by platinum at subsequent relapse.10 This strategy is supported by the results of the OVA-301 study (ClinicalTrials.gov identifier NCT00113607), which investigated trabectedin plus PLD in patients with relapsed, partially platinum-sensitive ovarian cancer.11 Several clinicians accept trabectedin plus PLD combination as an alternative to platinum-based regimens in this setting.
In general, platinum-resistant patients (those whose cancer recurs less than six months after the last platinum treatment) are treated with sequential, single non-platinum agents – such as paclitaxel, doxorubicin, etoposide or topotecan – rather than with combination therapy.4 These drugs are associated with a short-lasting response in 10–25 % of patients, who have a generally dismal prognosis.8,9
Novel treatment strategies are required to improve outcomes in women with recurrent ovarian cancer, particularly platinum-resistant disease. In this article, we look at one such option: the combination of a peptide containing the aspargine-glycine-arginine motif (NGR peptide) with human tumour necrosis factor (hTNF) plus doxorubicin.
Combination of an NGR Peptide and Human Tumour Necrosis Factor (NGR-hTNF)
TNF was first identified by Carswell et al. in the serum of Bacillus Calmette-Guérin-sensitised animals treated with an endotoxin that caused the release of a factor capable of inducing haemorrhagic necrosis of murine tumours.12 TNF has been shown to have cytostatic and cytotoxic effects against a wide range of human tumour cells and human tumour xenografts in nude mice.13,14 In addition to these properties, TNF has a broad spectrum of immunomodulatory activities,15 such as enhancing the expression of Class I major histocompatibility antigens on human endothelial cells, dermal fibroblasts and human tumour cell line16,17 as well as enhancing the expression of Class II major histocompatibility antigens on human T cells and tumour cells.18 TNF has also demonstrated multiple actions on natural killer cells, macrophage cells and granulocyte function.19–22 Moreover, there is evidence to suggest that the antitumour activity of TNF depends on indirect mechanisms associated with the selective obstruction and damage of tumour-associated blood vessels and on the activation of immune mechanisms, rather than on a direct toxic effect on tumour cells.23
Although numerous preclinical studies demonstrated that TNF has notable antitumour activity,12,14,22,24 early trials in humans indicated that its clinical use was limited by severe systemic toxicity.13,15,25–27 However, loco-regional therapy with high doses of TNF in combination with chemotherapy led to elevated response rates in patients with melanoma and sarcoma of the extremities27–31 and regression of bulky hepatic cancers confined to the liver30 and of peritoneal carcinomas.32
In vivo screening of peptide phage libraries has proven a powerful tool for discovering ligands that selectively bind to tumour vessels.33 Among these, an NGR peptide has been coupled to different anticancer compounds, such as doxorubicin and TNF, to enable the targeted delivery of these drugs to tumour vessels.23,33,34 Murine TNF (mTNF) was coupled to peptide CNGRC, an aminopeptidase ligand (CD13 antigen) capable of binding to tumour blood vessels.
This combination of an NGR peptide and mTNF (NGR-mTNF) was investigated in a series of preclinical experiments designed to measure its ability to induce the regression of neoplastic lesions, both as a single agent and in combination with chemotherapeutic drugs. These studies showed that NGR-mTNF is 12–15 times more effective than mTNF alone in suppressing the growth of RMA lymphomas and B16F1 melanoma in mice.23 The toxicity of the two treatments was of a similar magnitude. Modified hTNF was subsequently coupled to the CNGRC peptide sequence using recombinant DNA techniques.23 This combination of an NGR peptide and hTNF (NGR-hTNF) showed a similar potency differential to hTNF alone against RMA-T tumours in mice, suggesting that a similar effect, with an improved risk:benefit ratio, could be expected in human cancer treatment.
Anthracyclines are widely used chemotherapy agents in several types of solid and haematological tumours.35 It is claimed that they exert antitumour activity in ovarian cancer, but their effect on survival has only been demonstrated in isolated clinical trials. In most studies, in fact, the addition of doxorubicin had no effect on survival but did adversely affect the safety profile of the treatment, especially in terms of myelotoxicity. Some authors have reported that this scant response could be related to the poor penetration of doxorubicin into cancer cells.36 This, in turn, is probably related to the structural and functional abnormalities typical of the disorganised vasculature of tumour cells, as well as to the complete lack of lymphatics responsible for heterogeneous tumour perfusion, abnormal vascular permeability and increased interstitial pressure. These critical factors can limit the penetration of drugs into cancer cells distant from tumour vasculature and, consequently, the effectiveness of chemotherapy.36 Therapeutic strategies that enhance drug penetration have considerable potential to increase tumour sensitivity to cytotoxic drugs.
In pharmacokinetics studies, the dose-response curves for NGR-TNF and TNF alone (both given at 0.01, 1, 10, 100, 1,000 and 10,000 ng doses) indicated that 0.01 to 1 ng of NGR-hTNF per mouse (doses considered to be low) were sufficient to induce maximal effect (defined as tumour shrinkage). By contrast, in this experimental model, TNF was only active at high doses (1,000 and 10,000 ng per mouse).32 Interestingly, the dose-response curve for NGR-TNF as a single agent was bell-shaped; i.e., NGR-TNF induced tumour regression either at low doses (0.01–0.1 ng) or at high doses (1,000–10,000 ng). Other studies have shown that low doses of NGR-TNF (0.1 ng) synergistically enhanced the antitumour activity of chemotherapeutics such as doxorubicin,32 cisplatin and melphalan, with no evidence of increased toxicity. In particular, increased intracellular concentrations of doxorubicin in a higher percentage of cancer cells demonstrated that NGR-mTNF improved doxorubicin penetration in tumours.32 The delivery of minute amounts of TNF to tumour vessels represents a novel approach for avoiding negative feedback mechanisms and preserving the drug’s ability to overcome drug-penetration barriers. Such vascular targeting could represent a novel strategy for increasing the therapeutic index of chemotherapeutic drugs.
NGR-hTNF plus Doxorubicin
Preclinical Studies
The synergistic cytotoxic activity of NGR-mTNF plus doxorubicin has been reported in preclinical studies that showed increased tumour cell penetration by doxorubicin without changes in either the pharmacokinetic or toxicity profile of the drug.
In mouse models bearing WEHI-164 fibrosarcoma cells treated with NGR-mTNF (0.1 ng per mouse) followed by the administration of doxorubicin, maximum synergy was observed when doxorubicin was administered two hours after the NGR-mTNF infusion.37
A study in cynomolgus monkeys aimed to define the safety profile of NGR-hTNF plus doxorubicin. NGR-hTNF administered intravenously at 0, 50 and 1,000 ng per kg of body weight plus doxorubicin at 4 mg per kg of body weight every three weeks was well tolerated. Toxic effects considered to be related to doxorubicin were observed in the lymphopoietic system, at the injection site and in the lachrymal glands. No toxic effects or exacerbations of the doxorubicin-induced toxic effects attributable to NGR-hTNF were registered.38
Clinical Trials
In order to assess the safety profile of NGR-hTNF plus doxorubicin when administered sequentially in humans, a Phase Ib clinical trial (ClinicalTrials.gov identifier NGR003) was conducted in 15 patients, who received NGR-hTNF at escalating doses ranging from 0.2 to 1.6 μg/m2 plus doxorubicin at a fixed dose of 75 mg/m2.39 The most frequent NGR-hTNF-related adverse events (AEs) – i.e. those reported in at least 10 % of patients – were rigours, nausea, fatigue, hypertension, vomiting and pyrexia. Ten of the 15 patients (66 %) achieved disease stabilisation and two patients (13 %) achieved partial long-lasting (23 weeks) response. It should be noted that, of the 12 patients who achieved either stabilisation or partial response, nine had previously received anthracyclines and five had resistant disease. Overall, NGR-hTNF plus doxorubicin showed a favourable safety profile and encouraging cytotoxic activity, suggesting that the combination was suitable for further evaluation in Phase II studies.
A Phase II prospective study in patients with recurrent, resistant and partially platinum-sensitive ovarian cancer who had failed at least one previous line of platinum-based chemotherapy was conducted by our team at the Italian National Cancer Institute in Milan.40 Thirty-seven patients with radiologically documented progressive disease were enrolled and received NGR-hTNF plus doxorubicin, and the responses of 35 of them could be evaluated. Of those 35 patients, eight (23 %, 95 % confidence interval [CI] 11–40) achieved a partial response and 15 (43 %; 95 % CI 26–61) achieved disease stabilisation. Median progression-free survival (PFS) for the whole study population (n=37) was 5.0 months, but this increased to 8.2 months for patients who achieved partial response. Median PFS for patients who achieved disease stabilisation was 4.9 months. Among the 23 patients who fell into Gynecologic Cancer Intergroup (GCIG) CA-125 response criteria, nine responses (39 %) were recorded.
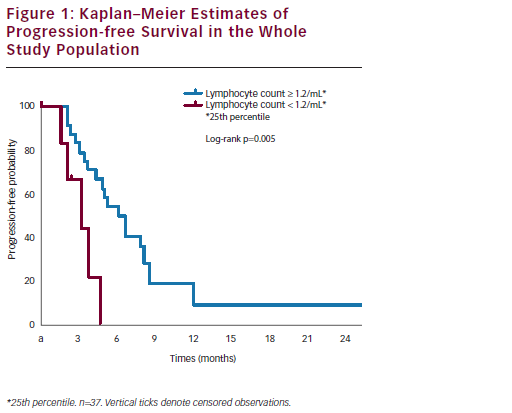
After a median follow-up of 14.6 months, the one-year survival rate was 65 %. Median survival was 24.0 months in patients with disease stabilisation or partial response and 4.9 months in patients with disease progression (p<0.0001) (see Figure 1). Interestingly, a correlation between baseline peripheral blood lymphocyte count (PBLC) and outcome was found. In the subset of refractory and/or resistant patients with a PBLC ≥1.2/ml (i.e., the 25th percentile distribution value), median PFS was 4.9 months, versus 2.6 months in refractory and/or resistant patients with a PBLC <1.2/ml (p=0.02). Median overall survival (OS) was 15.8 and 4.3 months in the two groups, respectively (p=0.0001) (see Figure 2). Univariate Cox analyses found that both longer platinum-free intervals (p=0.03) and higher baseline PBLCs (p=0.01) were significantly associated with longer PFS, while OS only correlated with higher PBLCs (p=0.001).40 Treatment appeared to be well tolerated. The most common Grade 3 non-haematological AE was asthenia (3 % of the 37 patients). Two patients (5 %) reported Grade 1 cardiac AEs (one tachycardia and one diastolic dysfunction). Only 9 % of all recorded AEs were related to NGR-hTNF infusion, and these AEs mainly consisted of transient infusion-related events such as Grade 1–2 rigours. Seven (19 %) and 16 (43 %) patients reported Grade 3 and Grade 4 neutropenia, respectively; nine (24 %) and one (3 %) patients reported Grade 3 and Grade 4 anaemia, respectively. Only one episode of neutropenic fever was reported (3 % of patients). All the drug cycles were given at the planned dose, except in eight patients (22 %) who required doxorubicin dose reductions.40
It was concluded that NGR-hTNF plus doxorubicin showed interesting clinical activity and a secure toxicity profile in recurrent ovarian cancer. It is noteworthy, particularly in the resistant and/or recurrent disease setting, that more than half of the patients in the study achieved and maintained control of their disease for a median period of more than six months. The findings that a low PBLC at baseline appeared to predict early progression and shorter PFS, and that normal PBLCs appeared to correlate with greater clinical benefits, are also interesting. These observations are consistent with the findings in immunocompetent nude mice lacking functionally mature T lymphocytes.40 In our study, baseline PBLC proved an easily accessible and reproducible predictive biomarker of outcome that could be exploited for selecting refractory and/or resistant ovarian cancer patients who would benefit the most from treatment with NGR-hTNF plus doxorubicin.
The toxicity profiles of the two elements of the drug combination did not overlap and the most commonly observed AEs were those expected when each is given alone (i.e., neutropenia with doxorubicin and rigours with NGR-hTNF). Previously reported cardiovascular toxicities associated with vascular targeting agents41 were not observed in this study40 and, more importantly, even though half of the patients completed six or more cycles of doxorubicin, increased cardiac toxicity usually associated with doxorubicin was not recorded.
Ongoing Trial
Based on the widespread use of doxorubicin in ovarian cancer, and the synergy between low-dose TNF and doxorubicin documented in preclinical models,42 a randomised Phase II trial (ClinicalTrials.gov identifier NCT01358071) testing doxorubicin with or without NGR-hTNF in recurrent, platinum-refractory and/or resistant ovarian cancer is currently ongoing across several Italian institutions. ■