Intraperitoneal Chemotherapy Employed as Primary Treatment of Advanced Ovarian Cancer
In early 2006, the third National Cancer Institutesponsored randomized phase III trial examining frontline regional cisplatin-based chemotherapy, compared with intravenous administration of the agent, was reported.1 As with the previous two studies,2-3 this trial demonstrated intraperitoneal delivery improved both time to disease progression and overall survival when employed as primary treatment of small-volume residual cancer following initial surgical cytoreduction (see Table 1). The finding led the National Cancer Institute to issue a Clinical Announcement to inform patients, physicians, and the public of the benefits of this management strategy in this clinical setting.4
Overall, the delivery of regional cisplatin-based chemotherapy reduces the risk of death by approximately 20–25% compared with an ‘all intravenous’ (IV) treatment program. It is important to note that the most recent phase III trial revealed the side effect profile of cisplatin-based intraperitoneal treatment to be greater than that of IV cisplatin. However, in the three reported phase III trials there was no increase in treatment-related deaths associated with regional therapy. Further, while a formal prospective quality-of-like analysis performed as a component of the most recently reported study did reveal a greater negative impact of intraperitoneal therapy on this clinically relevant parameter during treatment, one year following the completion of therapy there was no difference in quality-of-life between the two study arms (regional versus systemic approaches).1
A number of reasonable strategies have been proposed to maintain the efficacy of regional treatment of ovarian cancer while reducing the side effects of this novel management strategy.These include:
• slightly reducing the dose of intraperitoneal cisplatin delivery (e.g. from 100mg/m2 to 75mg/m2);
• substituting carboplatin for cisplatin;
• placing the intraperitoneal catheter at a separate surgery from the procedure performed to cytoreduce the cancer; and
• delivering three to four cycles of regional therapy versus the previously routinely planned six cycles.
Based on available data, it is appropriate to propose several additional clinical settings were intraperitoneal drug delivery may be considered a rational management option for patients with ovarian cancer (see Table 2). It must be emphasized that while it is reasonable to consider management of a particular patient in one of these situations with a regional strategy (with satisfactory informed consent), only the results of a well-designed and -conducted randomized phase III trial can truly define the genuine utility of this novel approach in these settings. Clinical Utility of Anti-angiogenic Agents in Ovarian Cancer
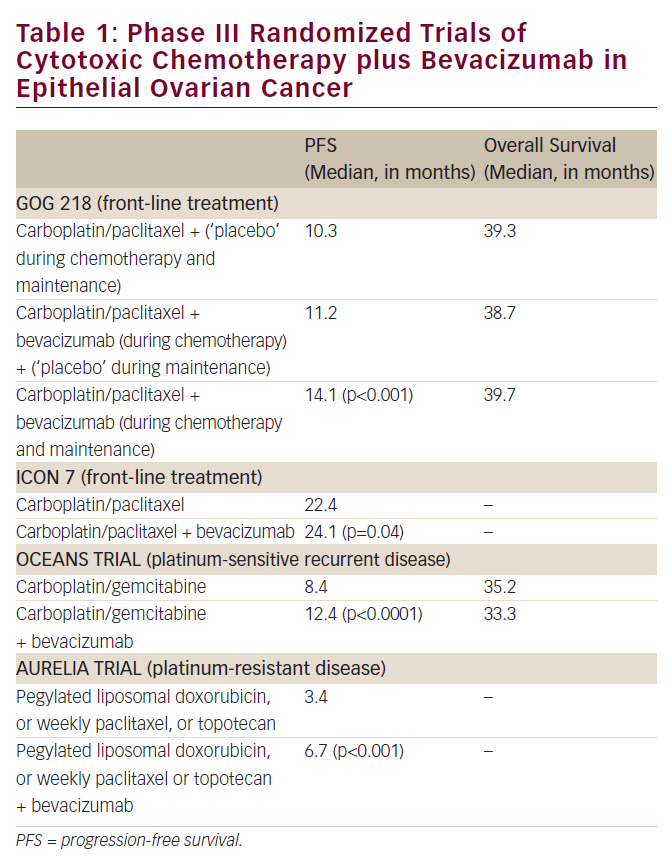
The favorable effects of bevacizumab, the first US Food and Drug Administration (FDA)-approved ‘antiangiogenic’ anti-neoplastic agent, in several malignancies (e.g. cancer of the colon, lung, and breast) have been documented.5 Of particular interest is the fact the administration of this drug exerts a more impressive impact on the time to disease progression and survival in these tumor types than suggested by its ability to produce a measurable decrease in the size of mass lesions when delivered as a single agent.5
Thus, the finding in several studies that the administration of single-agent bevacizumab results in an approximately 15% objective response rate when delivered as a second-line strategy in ovarian cancer (including platinum-resistant disease) was surprising, and has led to the provocative hope this class of agents would be of even greater utility in ovarian cancer than previously observed in other tumor types.6,7 While small studies and retrospective reviews of institutional experience have suggested an even higher response rate when bevacizumab is combined with cytotoxic chemotherapy in patients with recurrent ovarian cancer,8 there are currently no randomized trial data to support this conclusion in this malignancy.
IV = intravenous, IP = intraperitoneal, Cis = cisplatin, Cyclo = cyclophosphamide, Pac = paclitaxel.
The initial enthusiasm for use of bevacizumab in ovarian cancer has been somewhat tempered by the observation in these early studies that approximately 10% of heavily pre-treated individuals administered the agent developed a bowel perforation.7 Limited data suggest the risk of perforation is greatest where there is extensive intra-abdominal cancer, possibly due to the fact the antiangiogenic agent interferes with critically important wound healing. It is likely this process is on-going at the microscopic level in such individuals in the presence of growing tumor in close contact with the bowel wall. Several groups are currently exploring (in randomized phase III trials) a role for bevacizumab in the primary treatment of advanced ovarian cancer, when combined with platinum–taxane chemotherapy. Until the results of such studies are available, use of bevacizumab, and other anti-angiogenic agents, should be considered investigational in the primary management of ovarian cancer, but (based on available data) an acceptable option when employed as a palliative second-line strategy.
Combination Chemotherapy in the Second-line Treatment of Recurrent Ovarian Cancer
One of the striking aspects of the management of ovarian cancer is the extensive evidence-based data (i.e. phase III randomized trials) supporting management options in the primary treatment setting, and the rather profound lack of such information to guide clinicians caring for patients who experience recurrence of the malignancy. Fortunately, recently available data has begun to fill this void.
ICON-4, a somewhat controversial trial, explored the utility of paclitaxel plus a platinum agent versus nonpaclitaxel containing platinum therapy, employed as second-line treatment of recurrent ovarian cancer (i.e., treatment-free interval following the completion of primary chemotherapy of at least six months).9 Most observers have interpreted this study to show the combination of carboplatin plus paclitaxel improves both progression-free and overall survival in this setting, compared with the administration of carboplatin alone (see Table 3). Unfortunately, there was a far greater risk of clinicallyn relevant peripheral neuropathy associated with the combination paclitaxel-containing program (20% versus 1%). The recent publication of a second multi-institution randomized trial, comparing a carboplatin plus gemcitabine regimen with carboplatin alone, provides evidence-based data supporting an alternative program with a different toxicity profile (see Table 3).10 In this study, patients receiving the combination strategy experienced an improvement in progressionfree, but not overall, survival compared with carboplatin alone. Of relevance, there was no difference in neuropathy between the single-agent and two-drug treatment programs.
These data provide support for a second platinumbased regimen that might be employed in the recurrent disease setting. It would be reasonable to suggest that patients for whom neuropathy is not a concern (i.e. no clinically relevant neurotoxic effects with primary chemotherapy), the paclitaxel-containing regimen would be most appropriate, since this strategy has been shown to favorably impact overall survival. However, if neuropathy was previously documented in an individual patient, the carboplatin gemcitabine program may be the most rational option.
The availability of additional evidence-based data will be critical in assisting oncologists as they attempt to provide recommendations to their ovarian cancer patients regarding management options in the presence of documented recurrent, persistent, or resistant disease.
‘Maintenance’ Therapy in Ovarian Cancer
With the recognized substantial risk of relapse following primary treatment of advanced ovarian cancer, despite the high objective response rate to platinum-based chemotherapy, it was natural that investigators would explore the potential utility of continuing treatment beyond the ‘standard’ five or six cycles of therapy.A multiinstitution phase III trial revealed that continuing singleagent paclitaxel on a monthly schedule for a maximum of 12 months in patients who have achieved a clinically defined complete response to primary platinum-based chemotherapy substantially prolongs the time to subsequent disease progression.11 Unfortunately, this study was unable to firmly document an improvement in overall survival associated with this management strategy, potentially due to the fact the trial was discontinued by its Data Safety and Monitoring Committee when the profound improvement in progression-free survival was noted at a planned interim analysis. Of interest, a retrospective exploratory analysis performed following completion of this trial revealed superior overall survival for the patients treated with 12-monthly paclitaxel cycles who had initiated the maintenance treatment program with a serum CA-125 <10units/ml.12 An important confirmatory trial, being conducted by the Gynecologic Oncology Group, which again examines the impact of maintenance taxane therapy on survival in advanced ovarian cancer is in progress.
Chemotherapy in the Management of Endometrial Cancer
The role of chemotherapy in treatment of endometrial cancer has remained poorly defined. In fact, until relatively recently, oncologists were advised to routinely employ a progestational agent prior to administering a cytotoxic agent, due to the general belief such drugs possessed limited activity in this malignancy. However, data from both phase II and phase III studies revealing substantial biological activity for several combination chemotherapy regimens (e.g. carboplatin/paclitaxel; cisplatin/doxorubicin/paclitaxel; cisplatin/doxorubicin) has demonstrated the utility of such programs to achieve objective tumor regressions.13-14 Further randomized trials have confirmed the potential impact of chemotherapy on survival in metastatic or recurrent disease,14 and the superiority of cytotoxic drugs, compared with external beam radiation, in stage III endometrial cancer.15
While there remains an important role for hormonal therapy in metastatic endometrial cancer in the presence of specific characteristics of the disease process (e.g. low grade malignancy, presence of progesterone receptors, long interval between diagnosis and documented presence of metastatic cancer), chemotherapy is increasingly utilized in this malignancy. Defining an ‘optimal’ regimen in this cancer remains an important goal for future randomized trials.Advances in the Utility of Chemotherapy in the Treatment of Metastatic Cervix Cancer
The importance of combining cisplatin with radiation in the management of locally advanced cervix cancer has been well-recognized since the publication of a series of phase III trials demonstrating the favorable impact of this approach on overall survival compared with delivery of external beam radiation alone.16 Of interest,more recently available data has shown that several combination chemotherapy regimens, employed as therapy of metastatic or recurrent cervix cancer, can result in a superior outcome compared with the use of single-agent cisplatin (see Table 4).17-18
An on-going trial being conducted by the Gynecologic Oncology Group in the US is attempting to define the most appropriate combination chemotherapy regimen to utilize in this difficult clinical setting, where the current realistic goals of treatment are to modestly improve survival and improve cancer-related symptoms, while at the same time not negatively impacting overall quality of life associated with the toxicity of the systemic therapeutic program.
Cervical Cancer—The Future
There can be little doubt that one of the most important advances in the management of malignant disease over the past two decades has been recently published data from several randomized trials which reveal that two different human papillomavirus (HPV) vaccination strategies appear to be remarkably effective in both preventing persistent HPV infection (in women who do not have a pre-existing infection) and the development of cervical abnormalities recognized to be precursor lesions for invasive cancer.19–22
It is reasonable to speculate that the successful implementation of mass HPV vaccination strategies in both the developed and developing countries will result in a substantial reduction in the incidence of cervix cancer, and the truly profound suffering caused by this disease, within the next several decades.