RELATIVITY-047 reported on the initial efficacy and safety of relatlimab (RELA), an antibody to lymphocyte-activating gene 3 (LAG-3), in combination with nivolumab (NIVO), an antibody to programmed cell death 1 (PD-1).1 These positive findings add to the existing evidence for the efficacy of anti-PD-1 therapy – either alone or in combination with ipilimumab (IPI), an antibody to cytotoxic T-lymphocyte-associated antigen 4 – for patients with advanced melanoma. In the CheckMate 067 trial, combination NIVO and IPI demonstrated plateaus in progression-free survival (PFS) (34%) and overall survival (OS) (49%), and a median treatment-free interval of 27.6 months at a minimum 6.5 years’ follow-up.2 NIVO plus IPI also produced considerable toxicity, with grade 3–4 treatment-related adverse events (TRAEs) in 59% of patients. Phase III studies of anti-PD-1 monotherapy with NIVO or pembrolizumab (PEMBRO) have demonstrated plateaus in PFS (23–28%) and OS (38–39%) at median follow-up of 5 years.3,4 NIVO or PEMBRO monotherapy was better tolerated than combination NIVO/IPI, with grade 3–4 TRAEs in 16–17% of patients. Despite these promising results, many patients exhibit primary or acquired resistance to anti-PD-1-based therapy or experience immune-mediated adverse events that limit its use.
LAG-3 is a transmembrane protein expressed on activated CD4+ and CD8+ T cells, where it acts as a competitive ligand for major histocompatibility complex II, leading to an exhausted T-cell phenotype.5 Preclinical studies have demonstrated that concurrent blockade of LAG-3 and the PD-1 pathway reinvigorates antitumour immunity through reactivating CD8+ effector T cells and upregulating inflammatory cytokines.6 A phase I/II study in patients with advanced melanoma who progressed on anti-PD-1/programmed death-ligand 1 (PD-L1) therapy demonstrated the combination of RELA with NIVO had an objective response rate (ORR) of 16% and a disease control rate of 45%, with grade 3–4 TRAEs occurring in only 9% of patients.7
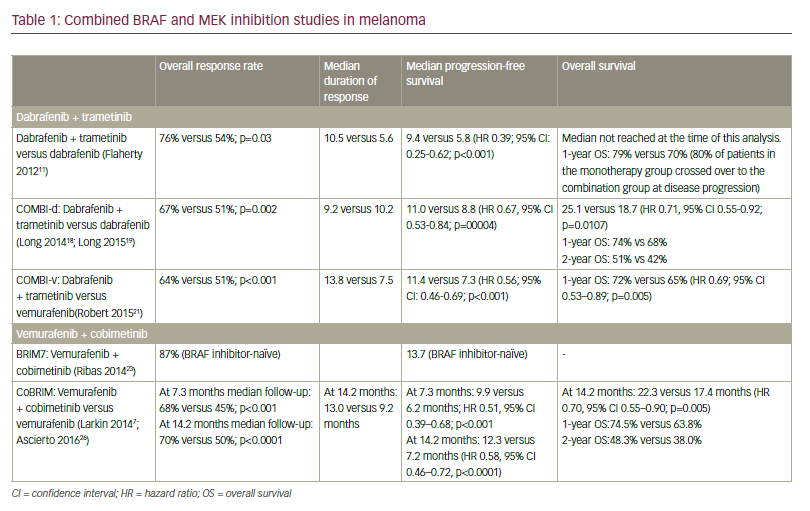
RELATIVITY-047 is a phase II/III trial for patients with treatment-naïve, unresectable or metastatic melanoma randomized to RELA 160 mg plus NIVO 480 mg, or NIVO 480 mg, given every 4 weeks.1 The study met its primary outcome with RELA plus NIVO demonstrating significant improvement in median PFS compared with NIVO monotherapy: 10.1 versus 4.6 months, respectively (hazard ratio [HR] 0.75, 95% confidence interval [CI] 0.6–0.9]; p=0.0055). Subgroup analysis demonstrated benefit regardless of BRAF mutation status, American Joint Committee on Cancer stage or LAG-3 expression. Interestingly, patients with tumour cell PD-L1 expression <1% showed greater PFS benefit from RELA plus NIVO than with NIVO alone (HR 0.66, 95% CI 0.51–0.84). The combination was associated with higher rates of grade 3–4 TRAEs than NIVO monotherapy (18.9% versus 9.7%, respectively). OS results were immature and ORR remained blinded at the time of presentation in accordance with the study’s planned hierarchical outcome assessment.
While these early outcomes demonstrate efficacy of RELA plus NIVO, several unanswered questions remain. First, RELATIVITY-047 was designed as a gated phase II/III study, resulting in follow-up ranging widely, from 1.3 to 33.1 months. As data continue to mature, particular interest will be given to whether the PFS benefit for RELA plus NIVO persists and extends to an OS benefit. Second, RELA and NIVO were administered as fixed-dose infusions given without interruption at 4-week intervals until progression or toxicity. Longer-term efficacy, toxicity and quality of life outcomes are necessary to understand how this combination, with its more protracted administration schedule, compares with induction NIVO plus IPI for up to 12 weeks followed by NIVO maintenance and, further, whether durable tumour response is possible without the need for ongoing treatment.8
RELA plus NIVO is an active regimen that will likely be used in the metastatic, neoadjuvant and adjuvant settings. In the neoadjuvant setting, RELA plus NIVO prompted pathological complete responses in 57% of patients with regional disease, a major pathological response rate of 66%, and no disease relapses in these responding patients at 16 months, thereby supporting the antitumour efficacy of this combination.9 No grade 3–4 adverse effects occurred prior to surgery, and occurred in 26% of patients on subsequent adjuvant RELA plus NIVO therapy. However, despite the impressive efficacy of RELA plus NIVO, based on the data to date NIVO plus IPI will likely remain the standard of care for patients with a BRAF mutation, high lactate dehydrogenase (LDH), or central nervous system or liver metastases, given the strong clinical data supporting this regimen in these patient subsets.10 Further, there may be an emerging role for reverse ratio NIVO 3 mg/kg plus IPI 1 mg/kg, which has demonstrated a significantly lower incidence of grade 3–5 TRAEs with no differences in efficacy endpoints compared with NIVO 1 mg/kg and IPI 3 mg/kg in some of these patient subsets. For patients without a BRAF mutation, normal LDH, or M1a/b disease, RELA plus NIVO may be favoured over NIVO monotherapy, particularly in those with tumour PD-L1 expression <1%. However, the current data suggest that anti-PD-1 monotherapy may be as efficacious as RELA plus NIVO for patients with tumour PD-L1 expression >1%. Given the heavy use of anti-PD-1 monotherapy in the adjuvant setting, studies of RELA plus NIVO for high-risk melanoma are particularly appealing, and are in the planning stages (NCT05002569).11
RELATIVITY-047 provides evidence supporting RELA plus NIVO as a breakthrough novel immunotherapeutic combination, producing a PFS benefit with adverse effect profile more favourable than NIVO plus IPI. Further studies investigating the ability of LAG-3 and PD-1 inhibition to reinvigorate exhausted T cells should be done in other advanced malignancies.