Chemoradiotherapy (CRT) has become a common treatment approach for patients with advanced head and neck cancer, particularly when the disease is unresectable or the loss of function from surgery is excessive. Following such treatment, the majority of patients show a complete clinical response in the primary site. Some of these patients do not have a complete pathologic response, however, and their subclinical disease is difficult to confirm unless the patient subsequently undergoes surgery. The development of positron-emission tomography–computed tomography (PET-CT) imaging represents an important advance in helping the clinician to determine which patients are most likely to have persistent cancer following CRT. Such information is critical to providing the optimal chance for disease control as this relates both to the tumor in the primary site and the regional lymph nodes.
Radiologic Interpretation of Positron-emission Tomography–Computed Tomography After Chemoradiotherapy
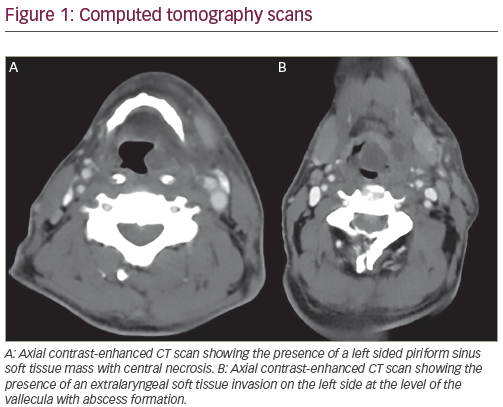
Combined PET-CT examination of patients after CRT allows the integration of both anatomic and structural information with functional metabolic activity in the assessment of persistent disease. The timing of the assessment is controversial, as metabolic tracer uptake and activity can be appreciated for weeks to months after CRT. Persistent disease, inflammation, superimposed infection, and radionecrosis can all potentially contribute to residual tracer uptake (see Figure 1). Proper interpretation needs to account for such post-treatment changes and appropriately distinguish them from persistent disease.
In a recent review of published literature assessing the utility of PET and combined PET-CT for monitoring patients after CRT, focal tracer uptake was the parameter generally utilized to discriminate between persistent disease and post-treatment changes.1 In general, focal asymmetric uptake within the primary site or lymph nodes, with intensity greater than the surrounding background and/or blood pool, should raise suspicion of potentially persistent disease. By contrast, post-treatment changes often appear non-focal and diffuse, conform to radiotherapy target volumes, and typically exhibit mild to moderate tracer uptake intensity.1 While much emphasis is placed on the qualitative interpretation and integration of PET-CT, the functional data obtained from PET-CT enables qualitative assessment of focal tracer uptake as well as quantitative analysis of metabolic activity. Despite this, such quantitative information, as measured by standard uptake values (SUVs) alone, may not be able to reliably and consistently differentiate tumor from inflammation. For example, a quantitative SUV threshold of 2.9 was shown by Yao et al. to identify residual nodal disease, but this value was not confirmed in subsequent studies in similar patient populations.2,3
Additional quantitative parameters currently being investigated may broaden the ability to delineate persistent disease as well as partial response. These include SUVLean and SUVLean Peak for more reliable and reproducible assessment of measurable tumor responses.4 Likewise, new parameters such as total metabolic tumor volume and burden may provide additional prognostic information.5–8
Prediction of Response to Chemoradiotherapy
An accurate and timely assessment of disease response after completion of CRT is critical for determining the need for surgical salvage versus clinical observation alone in patients with advanced head and neck cancer. As a result of better anatomic localization, combined PET-CT has fewer equivocal results and is more accurate than PET alone for the detection of head and neck cancer.9,10
The advantage of PET-CT over traditional imaging modalities, such as CT or magnetic resonance imaging (MRI), is its ability to provide physiologic information about potential regions of tumor metabolic activity in conjunction with anatomic localization. In addition, PET-CT provides easier access to whole-body information about the presence or absence of distant lesions (with lower radiation than CT and lower cost than MRI) that can affect prognosis and treatment. The ability of PET-CT to accurately predict disease response at the primary tumor site and cervical lymph nodes following CRT may prevent unnecessary surgical interventions designed to determine the completeness of non-operative treatment. This will lead to a potential reduction in patient morbidity and cost of treatment.
Assessment of the Primary Tumor Site
A limited number of studies have examined the utility of PET-CT specifically for the assessment of tumor response at the primary tumor site after CRT.3,11,12 There is substantial variability in the sensitivity and specificity of PET-CT for the detection of local disease based on these studies. The observed variations may be partially due to the time interval between completion of treatment and scanning. Similarly, a higher number of false-positive PET-CT scans are likely to be due to acute or chronic inflammation (see Figure 1). Inflammatory changes at the primary tumor site are typically related to recent treatment resulting in a relatively low positive predictive value (PPV) of PET-CT in detecting residual disease. The negative predictive value (NPV) of PET-CT at the primary tumor site is of much greater utility for confirming disease response. In multiple studies, the NPV of PET-CT for primary tumor control has been shown to be >90 %.3,11,12 A recent study on early (six- to eight-week) post-treatment PET-CT in patients treated with intra-arterial CRT revealed a NPV of 92% for the primary site. Ong et al. recently reported specificity and NPV of 95% and 97% respectively for PET-CT in the assessment of local disease in 65 patients treated with concurrent CRT for advanced squamous cell carcinoma of the head and neck.3 Only six patients had abnormal or equivocal [18F]-2-fluoro-2-deoxy- D-glucose–PET (18F-FDG-PET) uptake at the primary site at a median time of 12 weeks following treatment. None of these patients had local disease recurrence during the follow-up period. In a study by Chen et al. of patients with advanced oropharyngeal cancer treated with CRT, PET-CT performed an average of 7.3 weeks after treatment had a NPV of 95.7% in predicting residual tumor at the primary tumor site.12 Previous studies with PET alone have also been indicative of excellent NPV at the primary tumor site. A recent meta-analysis by Isles et al. using PET alone for detection of disease after radiotherapy or CRT reported an NPV of 95%.13
A negative post-treatment PET-CT at the primary tumor site provides a very accurate and reliable assessment of disease status. Absence of 18F-FDG-PET uptake in the primary tumor bed helps to confirm nonspecific abnormalities on CT scan, such as scarring or radiation changes that do not represent residual tumor. Consequently, a negative PET-CT may eliminate the need for evaluation and/or biopsy of the primary site under anesthesia to confirm the absence of disease and avoid the inherent risks of radionecrosis associated with biopsy of irradiated tissue. One can therefore suggest that panendoscopy and biopsy may be deferred in those patients with complete tumor response on clinical examination and a negative PET-CT after the completion of CRT.
Assessment of Cervical Lymph Nodes
The utility of PET-CT imaging for the assessment of residual neck nodal disease after definitive CRT has been an area of intense study and debate. Historically, in patients presenting with clinically advanced nodal disease (N2 or N3), the conventional approach has been to perform a planned neck dissection after completion of CRT, regardless of the extent of disease response in the cervical nodes.14–16 The rationale behind planned neck dissection in this group of patients was to remove any persistent nodal disease that could not be detected on physical examination or structural imaging studies and to confirm a complete pathological response.
Although still controversial, the treatment paradigm of routine planned neck dissection after CRT for patients with advanced nodal disease is beginning to change. Evidence from recent studies suggests that posttreatment neck dissection may not be necessary in patients with advanced neck disease achieving a complete clinical response after CRT based on physical examination and imaging studies that include the use of PET or PET-CT.3,11,17–19
At present, there have been no randomized clinical trials evaluating the effectiveness of PET or PET-CT for the assessment of nodal response after CRT. Data derived from retrospective studies, however, provide a valuable insight into the utility of this imaging modality for the prediction of disease response and patient management.
Similar to assessment of the primary tumor site, studies have shown significant variability in the PPV of PET or PET-CT for predicting persistent or recurrent nodal disease with PPV ranging from 18.2 to 91%.3,11,12,17,20–22 The exact reason for such wide ranges in PPV for the neck in is unclear. It may be related to the lack of consistent timing of posttreatment PET-CT, both within and between different studies, or the use of combined PET-CT versus PET alone.
Conversely, the real value of post-treatment PET-CT rests in its negative findings. In a meta-analysis by Isles et al. for PET alone, the pooled mean NPV for the detection of persistent or recurrent disease was 96%.13 In individual studies utilizing PET-CT surveillance after CRT, the NPV ranged from 94 to 100% in predicting nodal response and corroborating the earlier data from PET alone.3,11,20,23 The high NPV of PET-CT for detection of persistent nodal disease after CRT is resulting in a significant change in patient management away from routine planned neck dissection and towards a more conservative approach based on clinical and radiologic response to treatment. A variety of algorithms have been proposed for the assessment of patients after completion of CRT that incorporate the use of functional imaging (PET), structural imaging (CT and MRI), and routine physical examination.1–3,24 The overall approach of such algorithms includes early (four to six weeks) assessment of clinical response by physical examination.
Imaging utilizing PET-CT and contrast-enhanced CT or MRI scan is obtained six to 12 weeks after treatment. Patients achieving a complete clinical response based on physical examination and imaging studies may be observed (see Figure 2), whereas those patients with progressive disease or palpable and/or PET-positive disease are considered for surgical intervention.
Patient examination, interpretation of radiological studies, and subsequent management are best performed in a multidisciplinary setting. All patients should receive ongoing, routine follow-up for tumor surveillance. Timing of Positron-emission Tomography– Computed Tomography and the Need for Surgical Intervention
The appropriate timing of PET-CT after CRT is also an area of ongoing investigation and debate. The optimal time interval between completion of CRT and PET-CT imaging should result in an accurate assessment of disease response in a timely manner. This will direct appropriate surgical intervention, if needed, or allow for conservative clinical observation.
PET-CT scans performed too early after treatment may produce a high rate of false-positive studies due to treatment-related inflammation. Conversely, persistent disease below the level of detection for PET-CT may produce false-negative results if scanning is carried out too early. A recent study performed by a multidisciplinary head and neck oncology team demonstrated an overall accuracy rate of only 60% at the primary site for PET-CT performed between six and eight weeks after the completion of CRT.11 The NPV for the primary site as well as for the cervical nodes was, however, highly predictive of adequate disease control in this study. A protracted time interval between CRT and surgical intervention may delay the treatment of persistent disease or increase morbidity related to surgery after the onset of fibrosis in the neck.
An initial meta-analysis of the role of PET alone for detecting residual or recurrent head and neck cancer demonstrated greater sensitivity for scans performed 10 or more weeks after treatment.13 With the improved accuracy of combined PET-CT over PET alone, recent studies utilizing the combined imaging modality have demonstrated that a negative scan performed within six to eight weeks of completion is highly accurate (NPV 94–100%) for cervical lymph nodes.11,23 Other investigators recommend waiting until approximately 10 to 12 weeks after CRT in order to optimize PET-CT sensitivity and specificity, while still allowing adequate time for surgical intervention before the onset of extensive tissue fibrosis.1,3,13,20 Wide variability in the timing of scanning within each of these studies may affect the overall predictive values for post-treatment PET-CT. The authors recommend an algorithm for patient management after CRT that comprises physical examination four to six weeks after completion of treatment to assess initial disease response, followed shortly thereafter by imaging with contrast-enhanced CT and PET-CT (see Figure 3). In order to prevent an unnecessary delay in identifying and treating residual disease, imaging should be performed no later than 12 weeks after the completion of treatment, with a preference towards earlier imaging (at eight weeks).
If the results of the physical examination or the imaging studies are suggestive of persistent or progressive disease, appropriate surgical intervention should be performed. Ideally, the assessment of treatment response should be performed in a collaborative environment with input from the patient’s head and neck surgeon, medical oncologist, radiation oncologist and radiologist. The multidisciplinary setting provides the best context for interpreting the extent of disease response based on clinical and radiologic findings. It also enables the team to determine the direction for further patient management.
Conclusion
CRT for organ preservation in patients with advanced-stage head and neck cancer produces an excellent locoregional response rate. The addition of functional imaging (PET) to the traditional structural imaging methods (physical exam, CT, and MRI) has substantially improved the management of patients with advanced-stage head and neck cancer after CRT. It has also reduced the morbidity associated with routine surgical intervention regardless of clinical response.
The use of PET-CT with its high NPV has become a reliable tool for helping to determine which patients have achieved a complete tumor response at the primary tumor site and cervical lymph nodes. Patients with a negative post-treatment PET-CT and no evidence of disease on physical examination should be considered for observation rather than planned neck dissection.
There is some disagreement among investigators as to the exact timing of PET-CT after the completion of CRT. Although early PET-CT (after six to eight weeks) maintains a high NPV, there is a higher likelihood of false-positive findings and thus a lower PPV than scans performed longer (12 weeks) after treatment.
Ultimately, clinicians must strive to achieve a balance between timely and accurate assessments of tumor response and preventing the risks associated with delaying the treatment of persistent disease.