Nearly 12% of women newly diagnosed with breast cancer are younger than 45 years and most are candidates for adjuvant chemotherapy, hormone therapy or both.1 Many of these patients develop early menopause as a consequence of these treatments. Thus, infertility and premature menopause are relevant concerns for young women with cancer and may also influence their treatment compliance.2 Cancer treatments can temporarily or definitively damage the ovarian function, through the partial or total destruction of the ovarian reserve. Chemotherapy may have a direct effect on primordial oocytes, causing apoptosis or indirectly damaging the granulosa cells and the ovarian stroma.3–5
The probability of ovarian dysfunction after chemotherapy depends on the specific drugs used for the treatment, the dose, the schedule, the duration of the treatment, the age of the patient and her pre-treatment fertility status. The risk of permanent amenorrhoea is high with alkylating agents, intermediate with anthracyclines and taxanes and low with 5-fluorouracil and methotrexate. In particular, the combination regimen of oral cyclophosphamide, methotrexate and 5-fluorouracil (CMF) is associated with the highest risk of ovarian failure, which can reach a figure of 90 % in women older than 40.6 Modern combination regimens with anthracyclines and taxanes have less ovarian toxicity, with 85 % of patients under 40 resuming menses within 1 year after chemotherapy.7,8 Women under 35 have a 10 % higher risk of premature menopause, a 50 % higher risk between 35 and 40, while women over 40 have an 85 % higher risk.9 Young patients have a higher absolute number of primordial oocytes. Nonetheless, young patients face a sharp reduction of the ovarian reserve after toxic chemotherapy, and it has been demonstrated that menopause occurs earlier in cancer survivors.10,11
The use of tamoxifen at the end of chemotherapy contributes to delay the resumption of menses. Recent data support a longer duration of hormonal therapies for oestrogen receptor-positive breast cancer;12,13 therefore, larger numbers of women could be affected by the risk of compromised fertility. These women will be older and thus at higher risk for infertility at the time that their hormonal therapy is completed. No ovarian toxicity has been reported even after prolonged use of trastuzumab.14 It is well known that menstruation alone does not accurately reflect true ovarian function; measurement of anti-Mullerian hormone coupled with ultrasound-assisted antral follicle counting has been suggested as an effective mean of assessing the ovarian reserve following breast cancer chemotherapy.15
Use of GnRH Analogues to Gonado-protective Purpose During Chemotherapy
In recent years, much effort has been made to evaluate the role of administering gonadotropin-releasing hormone (GnRH) analogues to prevent premature ovarian failure and infertility. Several hypotheses, which are still the object of validation, have been formulated to explain this potentially beneficial effect for minimising the gonadotoxic effect of chemotherapy.16
Interruption of Follicle-Stimulating Hormone Secretion
Alkylating agents may bring about an increased rate of destruction/ apoptosis of non-resting follicles, and subsequently a decrease in the secretion of sex steroids. The resultant decrease in their plasma concentrations and the negative feedback on the hypothalamus and pituitary gland cause an increase in follicle-stimulating hormone (FSH) secretion, with a great recruitment of preantral follicles to enter the oneway differentiational path of maturation. Since the follicles are further exposed to the gonadotoxic effect of chemotherapeutic agents, there is an increased, rapid depletion of the ovarian follicular reserve. This vicious cycle may be interrupted by the GnRH analogue administration through its ability to prevent the increase in FSH concentrations.
Decrease in Utero-ovarian Perfusion
High oestrogen concentrations significantly increase ovarian perfusion. The decrease in utero-ovarian perfusion, resulting from the hypo-oestrogenic state generated by GnRH analogue pituitary-gonadal desensitization, may result in a lower total cumulative exposure of the ovaries to chemotherapeutic agents and subsequently in less gonadotoxicity.
Activation of GnRH Receptors
It has been shown that primate and human gonads contain GnRH receptors. GnRH analogues could activate GnRH-I and GnRH-II receptors, resulting in decreased follicular apoptosis.
Up-regulation of Sphingosine-1-Phosphate
Another possibility is that GnRH analogue may up-regulate an intragonadal antiapoptotic molecule such as sphingosine-1-phosphate. It has been proved that this molecule is able to prevent oocyte death caused by doxorubicin and ionising radiations. Protection of Undifferentiated Germ Line Stem Cells In 2004, ground-breaking data were presented regarding the existence of germ line stem cells in the mouse ovary, which are able to regenerate the primordial follicle pool. One can hypothesise that the GnRH agonist protective effect possibly takes place through the protection of the undifferentiated stem cells, which ultimately generate de novo primordial follicles, thus replacing the ones destroyed by chemotherapeutic agents.
Clinical Use of GnRH Analogues
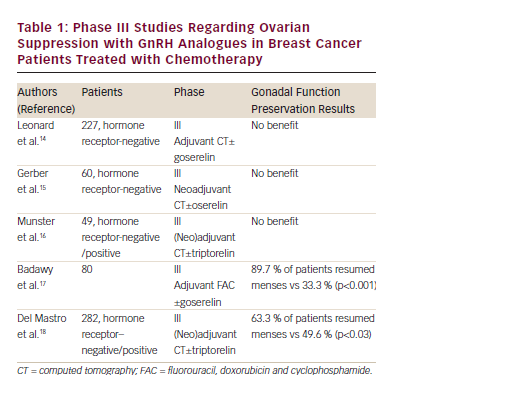
Prevention of premature menopause and infertility are major concerns for young patients who are undergoing treatment for cancer. The possibility of administering a GnRH analogue to prevent chemotherapyinduced gonadal damage is attractive, although the data are derived only from studies in animal models. The first results arising from phase II studies have suggested a reduction of the incidence of amenorrhoea with the use of GnRH analogues during chemotherapy in young women. However, the subsequent phase III studies conducted on premenopausal patients with early-stage breast cancer undergoing (neo)adjuvant chemotherapy, have produced conflicting data (see Table 1).
Leonard et al. have reported preliminary data from the Ovarian Protection Trial in Estrogen Negative (OPTION) study. This was a randomised, multicentric, open-label trial conducted on 227 premenopausal patients with primary hormone receptor-negative breast cancer undergoing anthracycline/cyclophosphamide (with or without taxane) based adjuvant chemotherapy for at least six to eight cycles. The patients were randomised to receive chemotherapy with or without goserelin. The primary objective was to determine the impact of goserelin on the incidence of premature ovarian failure following chemotherapy. The results did not show any difference in the two groups.17
The results of the Zoladex Rescue of Ovarian Function (ZORO) study published in 2011 did not show any benefit of using a GnRH analogue for preventing premature menopause during chemotherapy. In this prospective, randomised, open-label, controlled, multicentre study, 60 premenopausal patients with hormone-insensitive breast cancer were allocated to receive anthracycline/cyclophosphamide (with or without taxane)-based neoadjuvant chemotherapy with or without goserelin at the dose of 3.6 mg subcutaneously at least 2 weeks before the first chemotherapy cycle and then every 28 days until the end of the last cycle. The primary objective was the resumption of normal ovarian function, defined as two consecutive menstrual periods at 6 months after the end of chemotherapy. No significant difference was observed in the return of menses. The menstruation rates in the goserelin and observation arms at six months after the end of chemotherapy were 70 % and 56.7 %; a difference of 13.3 %, (p=0.284). At month 12 after the end of chemotherapy, the luteinising hormone (LH) levels in the control group were significantly higher (p=0.015) compared with those in patients receiving a GnRH analogue. The FSH levels, a more sensitive indicator of premenopausal status, in the control group were higher, but not significantly.18,19 These results may support the presence of a positive effect on the preservation of ovarian function in the group receiving the GnRH analogue.20 Furthermore, in the control group at 24 months, only 3 % of the patients developed chemotherapy-induced amenorrhoea. A possible explanation could be the use of a low gonadotoxic chemotherapeutic protocol and therefore this could be the reason for the negative results obtained in this trial. As such, the GnRH analogue cannot reach a significant difference between GnRH treated or untreated women.20,21
The findings are consistent with those published by Munster in 2012. This trial enrolled 49 premenopausal patients with newly diagnosed early-stage breast cancer (stage I to III) with oestrogen receptorpositive or negative. The patients were randomised to receive anthracycline/cyclophosphamide (with or without taxane)-based (neo)adjuvant chemotherapy with or without triptorelin at the dose of 3.75 mg by intramuscular injection starting least 1 week before the start of chemotherapy and then every 4 weeks for all the duration of chemotherapy. Women with oestrogen receptor-positive tumours were offered adjuvant tamoxifen therapy. In contrast to previous studies, women were stratified by age, oestrogen receptor status, hormonal therapy use and chemotherapy regimen. The primary objective of the study was to determine the effect of ovarian suppression by the use of GnRH analogue on the preservation of ovarian function as measured by the number of patients with restored menses during a follow-up period of at least 2 years after the last cycle of chemotherapy. Menses resumed in 90 % of the women in the control group and 88 % of the women in the triptorelin group (p=0.36). The premature end of the study could have led to its inaccurate conclusion. The study was targeted for 124 patients but was stopped for futility after 49 patients were enrolled. So, as in the ZORO study, the use of low gonadotoxic regimens requires a greater number of patients.22,23 Another recently published study demonstrated that GnRH analogue cotreatment did not offer a significant protective effect on ovarian function in hormone-insensitive breast cancer patients undergoing cyclophosphamide-based chemotherapy. In this study, 100 women (aged 18–40 years), stratified in relation to the beginning of the chemotherapy (at the same time or later than 10 days after study inclusion) were randomised to receive either chemotherapy alone or chemotherapy after downregulation with GnRH analogue. The primary outcome was the rate of resumption of menstruation at 12 months after the end of chemotherapy. The results showed that there were no differences in menstruation resumption rates between GnRH analogue-treated patients and control group individuals, in either early (80 % vs 80 %; p=1) or delayed chemotherapy subgroups (80 % vs 84 %; p=0.71); in addition, no differences were found in hormonal and ultrasound markers.24
Positive results for GnRH-agonist administration originate from two studies, published in 2009 and 2011. In the first study, 80 women who were diagnosed with unilateral breast cancer, between the age of 18 years and 40 (in premenopausal status), were assigned randomly to receive combined GnRH agonist and chemotherapy with fluorouracil, doxorubicin and cyclophosphamide (FAC regimen) for six cycles or chemotherapy alone. Goserelin was administered 2 weeks before the initiation of chemotherapy, at a dose of 3.6 mg subcutaneously and then every 28 days for 6 months. The primary objective of this study was the return of spontaneous menstruation and ovulation, measured through serial ultrasound scans. Also hormonal changes (FSH, LH, oestradiol and progesterone levels) were measured during and after the course of treatment. The rate of return of spontaneous menstruation and ovulation within 3–8 months from the end of treatment was significantly superior in the study group (89.7 % and 69.2 %) than in the control group (33.3 % and 25.6 %). Moreover, the median FSH and LH concentrations, 6 months after completion of the GnRH agonist/chemotherapy in the study group, were significantly lower than in the control group, whereas serum progesterone concentration was higher in the study group.25 This is a positive study; nevertheless, it might useful to underline the small number of randomised patients and the absence of data about the patient hormone-sensitivity status and the use of subsequent tamoxifen. The second study is the largest phase III study to evaluate the role of GnRH analogues in preserving ovarian function.
In this study, 282 premenopausal women with stage I–III breast cancer, candidates for adjuvant or neoadjuvant chemotherapy (CMF, anthracyclines or anthracyclines and taxanes-based regimens), were randomly allocated to receive chemotherapy alone or combined with triptorelin, at a dose of 3.75 mg i.m. at least 1 week before the start of chemotherapy and then every 4 weeks for the duration of treatment. Patients with hormone-sensitive tumours also received tamoxifen 20 mg/day at the end of chemotherapy for 5 years and, in case of return of menses during the 12-month period of observation, they received in addition to tamoxifen, 3.75 mg of triptorelin every four weeks until ovarian function had been suppressed for at least 2 years. This strategy has been adopted to avoid the hypothesised detrimental effect of the lack of chemotherapy-induced amenorrhoea on the outcome of patients with hormone-sensitive tumours. The primary study objective was to compare in the two arms the incidence of chemotherapy-induced early menopause, defined as no resumption of menstrual activity and postmenopausal levels of FSH and estradiol for one year after the end of chemotherapy. This 12-month period of observation to define early menopause differentiates this study from previous studies where a shorter interval (3–6 months) was considered.
The intention-to-treat analysis showed a rate of early menopause of 25.9% in the chemotherapy-alone group and 8.9 % in the chemotherapy plus triptorelin group. The number needed to treat was six. Resumption of menses was observed in 49.6 % of patients in the chemotherapy-alone group versus 63.3 % of patients in the chemotherapy plus triptorelin group. The median time to resumption of menstrual activity was 6.7 months in the patients treated with triptorelin and was not reached in the patients treated with chemotherapy alone. The resumption of menses was higher in hormone receptor-negative patients who did not receive tamoxifen; these data confirm that the administration of tamoxifen after chemotherapy is associated with an increased incidence of amenorrhoea. There were no differences in the incidence of disease recurrences or deaths in the two arms.26 In addition to phase III studies, three meta-analyses have been conducted to evaluate the gonado-protective effect of GnRH analogues. The first meta-analysis was conducted by the Cochrane Collaboration in 2011. Data from only four studies were included, with a total of 157 patients affected by lymphoma and breast cancer. The results of this meta-analysis showed that the administration of a GnRH agonist had a protective effect on menstruation (resumed menses: relative risk [RR] 1.90 IC 1.30–2.79) and ovulation (RR 2.70 IC 1.52–4.79) after chemotherapy, although there were no significant differences in pregnancy rates between the groups.
The authors’ conclusion was that GnRH analogues appear to be effective in protecting the ovaries during chemotherapy in terms of resumption of menses, but evidence for protection of long-term fertility is lacking.27 In the second meta-analysis, data from eight randomised studies, involving 803 breast cancer and lymphoma patients randomly assigned to receive chemotherapy or chemotherapy and GnRH analogue, were collected. A pooled analysis of data showed a significant reduction of premature ovarian failure induced by chemotherapy with the administration of GnRH analogue (odds ratio [OR] 0.49; IC 0.35–0.69). However, because of the important heterogeneity of the studies included in the metaanalysis, the authors’ conclusion was that large and well-designed randomised clinical trials should be conducted to clarify the effects of GnRH analogues in preventing chemotherapy-induced ovarian failure.28 The more recently published meta-analysis is the most homogeneous since it included five randomised controlled trials enrolling 528 premenopausal early breast cancer patients, of whom 274 received GnRH analogue (goserelin or triptorelin) with chemotherapy and 254 received chemotherapy alone.29 The results demonstrated that administration of GnRH analogue was associated with reduced rates of premature ovarian failure (of note, the five trials used various definitions of premature ovarian failure) within 1 year after completion of the chemotherapy (RR 0.40; IC 0.21–0.75). However, during the longest follow up, the rates of menses resumption and of spontaneous pregnancy were not significantly different between the two arms. Although GnRH-agonist suppression of the reproductive axis causes typical symptoms of menopause, the metaanalysis did not find a significant difference in adverse events between the two groups.
Conclusion
Cancer treatments may temporarily or definitively damage the ovarian function. Premature menopause and irreversible sterility are the most dramatic outcome of ovarian dysfunction and these are associated with an impaired quality of life and influence patient’s treatment compliance. Discussions about fertility and fertility preservation are of great importance to patients with cancer. Hence the interest in identifying effective, tolerable, safe and non-invasive methods to prevent the consequences of premature menopause and, in particular, to preserve fertility in young women exposed to chemotherapy.
The role of temporary ovarian suppression with the administration of the GnRH analogue during chemotherapy has been evaluated in several studies from which are derived conflicting results.
Evaluating the results of these studies together is difficult because these are influenced by many factors: the different age of the study populations, the different type of chemotherapy used, the inclusion or exclusion of oestrogen receptor-positive patients and the treatment with tamoxifen, the different endpoints (amenorrhoea vs menstruation vs laboratory values) and the different timing of ovarian function assessment. In addition, the limited length of follow up in these studies preclude the possibility to determine the long-term impact of GnRH analogues on preservation of ovarian function and fertility. Another important aspect is the possible detrimental effect of the maintenance of ovarian function in patients with hormone-sensitive breast cancer. The available data show an improvement in survival in patients with hormone-sensitive breast cancer that achieve amenorrhoea with adjuvant chemotherapy. However, available data seem to rule out the possible negative interference of GnRH analogues on the effectiveness of adjuvant chemotherapy.30 Finally, a limitation to the use of GnRH analogue to preserve the ovarian function is the assumption that resumption of menses corresponds to retained fertility; these women may continue to menstruate after chemotherapy, despite having abnormal fertility. Since this has not been proved, other options should be considered, such as oocyte or embryo cryopreservation. These options are actually the only established fertilitypreservation methods: newer hormonal stimulation regimens (letrozole and tamoxifen) may be effective as traditional methods and their use may be preferred in women with hormone-sensitive cancer; letrozole, for instance, can enhance ovarian stimulation while keeping oestrogen levels near physiological levels.
Despite the attractiveness of the use of GnRH analogue in preventing ovarian failure for many advantages (simplicity in administration, no significant side effects, non-invasive method and the relatively low cost of the therapy), the question regarding its effectiveness, especially as method of fertility preservation, is still not resolved. Given the current state of knowledge regarding these agents, the recent update of American Society of Clinical Oncology (ASCO) clinical practice guidelines on fertility preservation for patients with cancer judged GnRH analogues, to date, as a not proved effective method of fertility preservation and stressed the need for a decisive study in which a larger number of more homogeneous patients will be randomised to a treatment with or without GnRH analogues, will be followed for a longer period of time and more sensitive ovarian reserve markers such as anti-Mullerian hormone or antral follicle counts will be used.31













