Cumulatively, TNBC accounts for 35–40% of breast cancer deaths in the United States, despite only comprising 10–15% of total breast cancer cases diagnosed.1 Even before the use of anti-estrogen therapies became widespread, hormone receptor-positive breast cancer (HR+BC) provided a far more favourable risk of recurrence and overall prognosis compared with TNBC.2,3 Once metastatic, TNBC historically carries a median overall survival (mOS) of roughly 18–25 months with the use of conventional cytotoxic chemotherapy.4,5 In light of this, breast cancer researchers, industry leaders and drug regulators, including the FDA, have focused on ways to streamline therapies and accelerate drug approvals, both for early- and late-stage TNBC.6,7 However, for the purpose of this review, we will be focusing primarily on the use of pembrolizumab in metastatic triple-negative breast cancer.
Programmed death-1, programmed death-ligand 1 and cytotoxic T-lymphocyte antigen 4 function
Programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA4) are inhibitory molecules located on the surface of circulating immune cells, including cytotoxic and helper T-cells.8,9 PD-1 binds to programmed death-ligand 1 (PD-L1) and 2 (PD-L2), whereas CTLA4 binds to ligands B7-1 and B7-2. Once bound to their appropriate ligands, these surface proteins are responsible for a signal cascade that ultimately leads to immunosuppression due to decreased immune-cell activation. Under normal physiology, PD-L1 is expressed by a variety of cell types, including those in the thymus, cornea, testis and placenta, to prevent autoimmune responses and to allow for the creation of immune-privileged regions.10,11 The expression of PD-L1 by malignant cells and surrounding tumour-infiltrating immune cells plays a similar role in that it enables cancer cells to elude the immune system in order to avoid destruction.12,13 PD-L2 is expressed predominantly on professional antigen-presenting cells, including macrophages and dendritic cells.14 PD-L2 is also expressed in the respiratory and gastrointestinal tracts, as well as in a wide variety of malignancies, including TNBC.10
Researchers have been able to overcome tumour immune evasion by creating ICIs, including anti-CTLA4 (ipilimumab), anti-PD-1 (pembrolizumab, nivolumab, cemiplimab, dostarlimab) and anti-PD-L1 (atezolizumab, durvalumab, avelumab) antibodies.15–17 These ICIs restore the immune system’s capability to induce apoptosis and release cytotoxic factors within the tumour. Additional ICIs remain on the horizon and are being fast tracked at a rapid pace.
Pembrolizumab
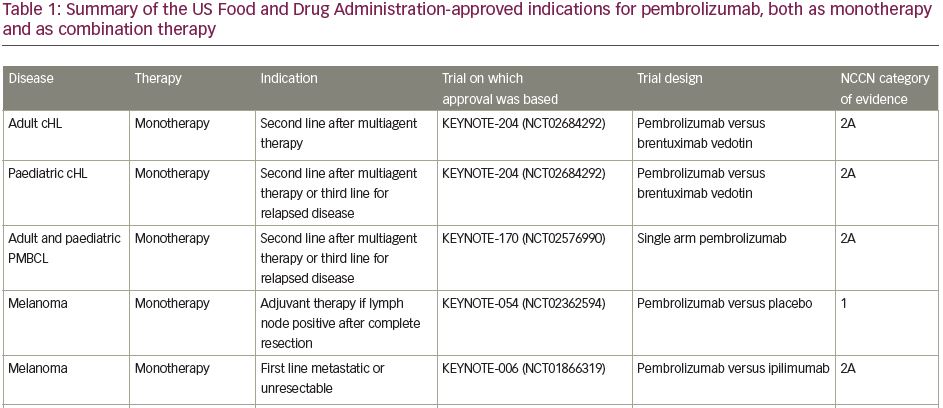
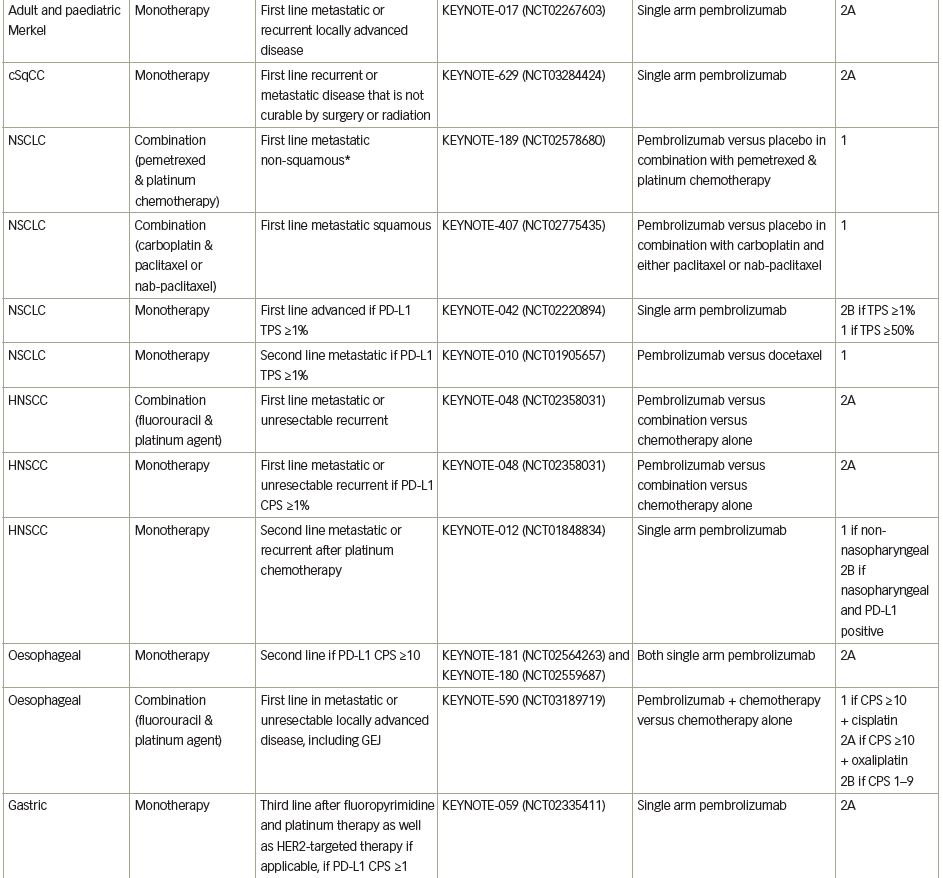
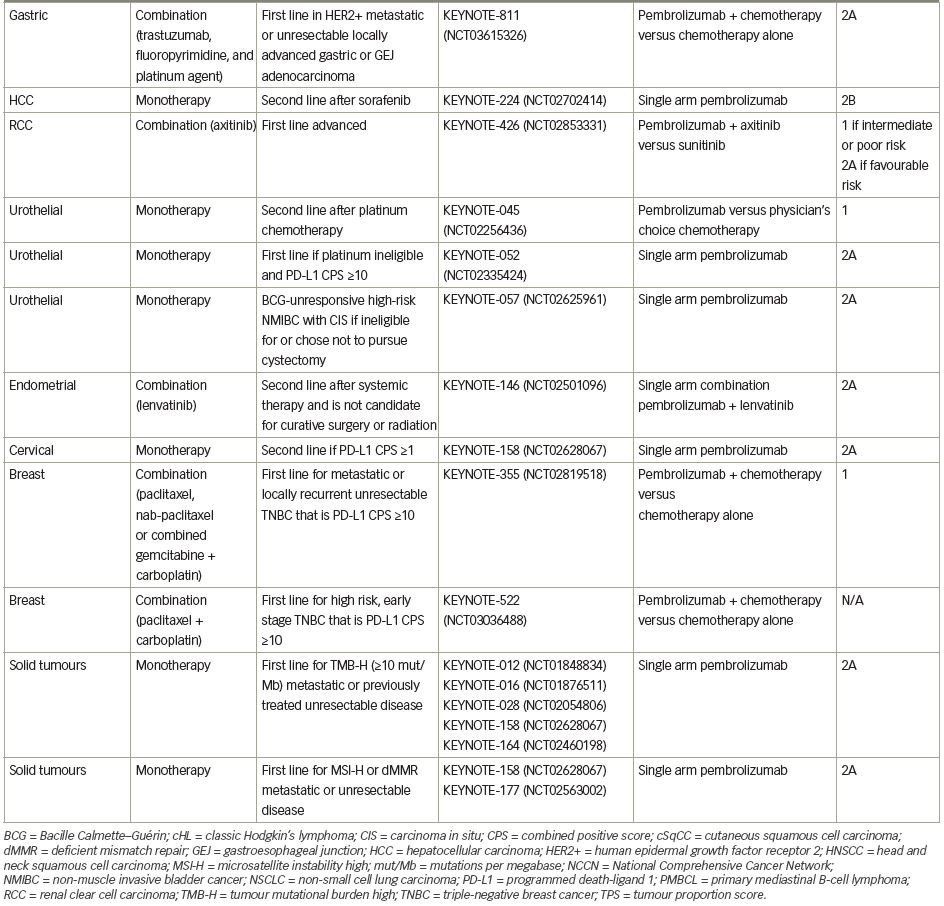
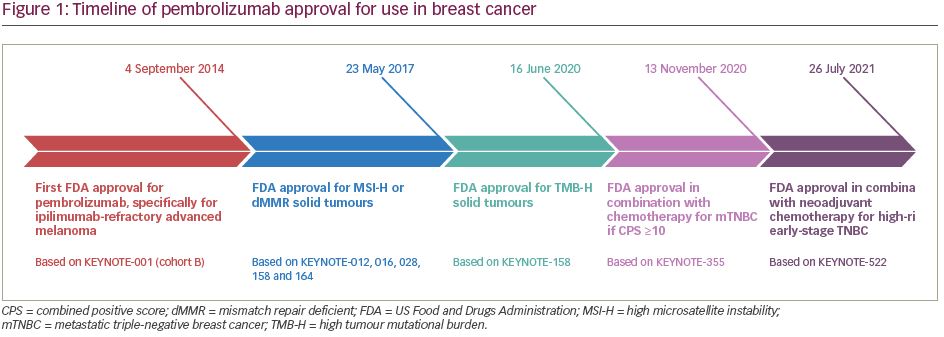
Pembrolizumab (previously MK-3475) is a human immunoglobulin G (IgG)4 kappa monoclonal antibody antagonist of PD-1. Manufactured by Merck & Co., the marketed version of the drug, Keytruda®, has a half-life of approximately 23 days.18 Pembrolizumab received breakthrough designation in 2013 and accelerated FDA approval in 2014 following the success of the KEYNOTE-001 trial in ipilimumab-refractory advanced melanoma.19 As of July 2021, pembrolizumab has 29 FDA-approved indications, making it acceptable for use as monotherapy or in combination with other agents (Table 1).20 More broadly, pembrolizumab has also received tissue-agnostic approval for use in solid tumours found to be microsatellite instability high (MSI-H; defined as ≥30% mutations), mismatch repair deficient (dMMR), or in solid tumours with a high tumour mutational burden (TMB-H; defined as ≥10 mutations/megabase).21,22 Even within the field of breast cancer, the indications for pembrolizumab continue to expand quickly. In July 2021, the US FDA granted accelerated approval of pembrolizumab for use in high-risk, early-stage TNBC when used in combination with neoadjuvant chemotherapy, followed by administration as adjuvant monotherapy based on the results of KEYNOTE-522.23 Further discussion of the use of pembrolizumab in the neoadjuvant setting is beyond the scope of this review. The timeline of pembrolizumab approval for use in breast cancer is illustrated in Figure 1.
High microsatellite instability and high tumour mutational burden
Certain features in solid tumour malignancies have been linked to increased ICI responsiveness, starting with tumour mutational burden (TMB). The increasing presence of somatic mutations in malignant cells leads to increased expression of neoantigens on their surface via major histocompatibility complex class I (MHC-1) proteins, resulting in higher rates of lymphocyte detection and activation.24 Another feature is microsatellite instability (MSI). Microsatellites are short tandem repeats of DNA found scattered across the genome that are prone to replication errors due to slippage by DNA polymerase, which in turn leads to the addition and/or removal of bases, causing the formation of mismatched DNA strands. These are typically repaired by mismatch repair (MMR) proteins, which include MLH1, MSH2, MSH6 and PMS2, but tumours deficient in any one of these proteins are unable to correct these errors, leading to MSI and the accumulation of somatic mutations with downstream expression of neoantigens.25 Large-scale clinical trials have since validated these findings, and demonstrated that higher response rates to pembrolizumab are observed in solid malignancies that are either TMB-H or MSI-H.21,22,26,27
Data pooled from five key, single-arm, multi-cohort studies (KEYNOTE-158,21 KEYNOTE-016,27 KEYNOTE-164,28 KEYNOTE-01229 and KEYNOTE-02830) were used by the FDA in 2017 to determine pembrolizumab efficacy in patients found to have MSI-H or dMMR tumours.18,21 Of the 149 patients found to have MSI-H or dMMR tumours at the time of review, 98.0% of them had metastatic disease, with a slim minority (1.3%) involving primary breast carcinomas. Collectively, the objective response rate (ORR) was 39.6% with a complete response (CR) rate of 7.4%. When examining the non-colorectal carcinoma category, ORR improved to 46.0%.
The 10 cohorts (A–J) of KEYNOTE-158 were also examined to determine pembrolizumab efficacy in patients with TMB-H non-colorectal tumours. 18,22 Similarly to the MSI-H studies mentioned above, these cohorts were designed as phase II, single-arm trials involving pembrolizumab therapy which was continued until disease progression or an unacceptable toxicity was observed. Of the 1,050 patients enrolled, only 102 (13.0%) had confirmed TMB-H tumours. The majority of patients had small-cell lung cancer (33.0%), cervical cancer (16.0%), vulvar cancer (15%) and anal cancer (14.0%). No breast cancer patients met TMB-H criteria. The ORR for TMB-H patients was 29.0% (versus 6.0% for non-TMB-H matched controls). When excluding those with concurrent MSI-H and TMB-H tumours (21.0% of the TMB-H patients), ORR was 28.0%.
Recent large-scale retrospective analyses have estimated MSI-H prevalence to be roughly 0.4% in TNBC, 0.2% in HR+BC and 0.1% in human epidermal growth factor receptor 2-positive breast cancer (HER2+BC), whereas TMB-H prevalence was estimated at 9% in TNBC, 8.0% in HR+BC and 12.0% in HER2+BC.31 Additionally, metastatic tumours confer higher rates of TMB-H prevalence compared with localized tumours (8.4% versus 2.9%), as do invasive lobular carcinomas over invasive ductal carcinomas (17.0% versus 7.8%).32 When comparing outcomes in patients found to be TMB-H, there is no clear correlation between increasing TMB levels and progression-free survival (PFS) with the use of pembrolizumab monotherapy. The recently reported TAPUR study demonstrated some signs of clinical efficacy with pembrolizumab monotherapy in TMB-H breast tumours, with a disease control rate of 37.0% and an ORR of 21.0%.33 Important to note is that responses were seen in TMB-H breast tumours irrespective of hormone receptor status.
Programmed death-ligand 1 expression
Immunohistochemistry (IHC) is used to quantify the cumulative surface expression of PD-L1 on both malignant cells and immune cells. For breast cancer specifically, the two most commonly used assays are Ventana SP142 (Roche, Basel, Switzerland) and Dako 22C3 (Agilent Technologies, Santa Clara, CA, USA).34,35 Ventana SP142 is the companion assay used for atezolizumab studies. It reports the percentage of PD-L1 expression seen on tumour cells and immune cells, with a positivity cut-off set at immune cells ≥1.0%.36 In contrast, Dako 22C3 is the companion assay used for pembrolizumab studies. It reports the cumulative expression of PD-L1 on tumour cells and immune cells to calculate a combined positive score (CPS) with a positivity cut off of ≥10 for pembrolizumab approval in TNBC.37–39 These assays are not interchangeable and have different cut-offs depending on the cancer type. The overall rate of PD-L1 positivity in TNBC ranges from 20−60% depending on the assays and methods used.40–42 Lower expression is seen in distant lesions involving the liver, bones and skin, whereas higher expression is seen in metastatic deposits in the brain, lungs and lymph nodes.43,44 High PD-L1 expression is also seen when tumours carry activating mutations in the oncogene phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), as well as in those with deleterious mutations involving the tumour suppressor gene phosphatase and tensin homologue (PTEN).45 Interestingly, these mutations confer resistance to checkpoint inhibitors across various solid malignancies, though concurrent use of PIK3CA inhibitors may sensitize tumour cells to immunotherapy.46 Using conventional chemotherapy, particularly paclitaxel, has also been shown to increase both mRNA and surface protein expression of PD-L1 in TNBC.47,48
Pembrolizumab monotherapy
KEYNOTE-012
KEYNOTE-012 was a phase Ib trial investigating the treatment activity and overall safety of pembrolizumab monotherapy in 27 patients with advanced PD-L1-positive TNBC.29,49 Pembrolizumab monotherapy produced an ORR of 18.5%, median-PFS (mPFS) of 1.9 months and mOS of 10.2 months. Given that most patients were heavily pre-treated (25% had received ≥5 prior lines of therapy), these efficacy results were an improvement compared with historic single-agent chemotherapy.5 In light of its promising anti-tumour activity and tolerable safety profile, further large-scale trials were conducted to better assess efficacy outcomes.
KEYNOTE-086
KEYNOTE-086 was a phase II trial with two cohorts. Cohort A evaluated pembrolizumab in patients who progressed on ≥1 prior metastatic therapy, and cohort B evaluated treatment-naïve patients with PD-L1-positive, mTNBC.50,51 The results of cohort A were released first with a reported ORR of merely 5.3% that was unaffected by PD-L1 expression (5.7% if PD-L1 positive and 4.7% if PD-L1 negative), along with an mPFS of 2.0 months and mOS of 9.0 months. However, for cohort B, the results were more promising, with an ORR of 21.4%, mPFS of 2.1 months, and mOS of 18.0 months. When compared with standard-of-care, single-agent chemotherapy, the results were positive and ultimately led to a head-to-head comparison trial, KEYNOTE-119.
KEYNOTE-119
The interim results of KEYNOTE-119 were released at the 2019 European Society of Medical Oncology (ESMO) Congress.52 KEYNOTE-119 was a phase III trial performed to determine if pembrolizumab monotherapy was superior to conventional chemotherapy (physician’s choice) in 622 patients with treatment-refractory mTNBC. For chemotherapy options, however, investigators’ choices were limited to either capecitabine, eribulin, gemcitabine or vinorelbine. The primary outcome of overall survival was stratified to evaluate patients with a CPS ≥10, CPS ≥1 and those in the intention-to-treat (ITT) population. After a median follow-up of 9.9 months in the experimental arm and 10.9 months in the control arm, mOS was equivalent between the ITT groups. However, mOS improved for the pembrolizumab arm as the CPS score increased, though the difference was statistically insignificant (ITT net -0.9 months, CPS ≥1 net +0.5 months, CPS ≥10 net +1.1 months). A post hoc exploratory analysis for patients with a CPS ≥20 (n=6) was later performed, which found that mOS was 14.9 months versus 12.5 months (hazard ratio (HR) 0.58; 95% confidence interval (CI) 0.38–0.88) in the control group. The main takeaway from KEYNOTE-119 was that pembrolizumab did not improve outcomes compared with single-agent chemotherapy. However, whether pembrolizumab monotherapy could be deemed a suitable alternative to chemotherapy in treatment-refractory mTNBC necessitates further research, as this study was not designed as a non-inferiority trial.
Pembrolizumab combination therapy
TOPACIO: pembrolizumab + niraparib
Niraparib inhibits poly(adenosine diphosphate ribose) polymerase (PARP), an enzyme important for repairing double-stranded DNA breaks, and is efficacious when used against tumours deficient in DNA repair enzymes, specifically those with mutations of either breast cancer gene type 1 (BRCA1) or type 2 (BRCA2). TOPACIO was a single-arm phase II trial that combined niraparib with pembrolizumab in patients with advanced TNBC, regardless of their BRCA status, with the primary outcome of ORR.53 Of the 47 patients who had available efficacy data, 15 (32%) had tumour-mutated BRCA, and 28 (60%) were found to be PD-L1 positive, though certain patients were untested among the total group. Regarding outcomes, the overall ORR was 21%. Among PD-L1-positive patients, the ORR was 32% (versus 8% in PD-L1 negative), whereas among BRCA-mutated patients, the ORR was 47% (versus 11% in BRCA wild-type). Overall, the combination therapy appeared to be auspicious, but given the small sample size, limited conclusions could be drawn.
Pembrolizumab + capecitabine
Capecitabine is a pro-drug of 5-fluorouracil that works by inhibiting thymidylate synthase. This prevents the production of thymidine, which is essential for DNA synthesis. A phase II trial assessed the combination of pembrolizumab with capecitabine in patients with non-HER2-expressing breast cancers.54 Of the 30 patients enrolled, 16 had mTNBC. In all patients, the ORR was 14% (13% for TNBC), mPFS was 4.0 months (4.0 months for TNBC) and mOS was 15.4 months (15.3 months for TNBC). The trial was adequately powered but failed to demonstrate a meaningful improvement in PFS compared with historic controls. PD-L1 positivity was observed in 40% of all patients, but calculated at 60% in the TNBC subgroup.
ENHANCE: pembrolizumab + eribulin
Eribulin works primarily as a microtubule inhibitor and is typically reserved for treating metastatic breast cancer in patients previously treated with taxane and anthracycline therapies. ENHANCE was a single-arm, phase Ib trial that enrolled 107 patients with mTNBC with ≤2 prior lines of systemic therapy regimens, and tested the combination of pembrolizumab and eribulin.55 The phase Ib run-in with six patients found the recommended phase II doses to be eribulin 1.4 mg/m2 on Days 1 and 8 of the 21-day cycle and pembrolizumab 200 mg on Day 1. For phase II of the trial, the resulting ORR was 23.4% with an mPFS of 4.1 months and mOS of 16.1 months. Patients were stratified based on their current line of therapy and PD-L1 positivity. While there was a trend towards improved outcomes in those who were PD-L1 positive and on first-line therapy, the study was too small to make any definitive claims.
KEYNOTE-355
KEYNOTE-355 was a large phase III, double-blinded, international, placebo-controlled trial in which 847 patients with advanced TNBC were assigned 2:1 to receive either pembrolizumab or placebo in addition to conventional frontline chemotherapy.56 Physician’s choice for conventional chemotherapy included paclitaxel (90 mg/m² on Days 1, 8 and 15, every 28 days), nab-paclitaxel (100 mg/m² on Days 1, 8 and 15, every 28 days) or combination gemcitabine/carboplatin (gemcitabine 1,000 mg/m² plus carboplatin with an area under the curve of 2 on Days 1 and 8, every 21 days). Pembrolizumab/placebo was given at a standard dose of 200 mg intravenous (IV) every 21 days. The primary outcomes were initially designed to stratify overall survival and PFS between the ITT population and patients with CPS ≥1, but following newer biomarker outcome data, the investigators further subcategorized patients with a CPS ≥10, as this cohort was likely to show improved outcomes.57 During the release of the second interim results, analysis of overall survival was still ongoing but the final assessment of PFS was completed with a median follow-up of roughly 26 months for each arm. A significant increase in mPFS was observed in the CPS ≥10 experimental arm at 9.7 months versus 5.6 months (p=0.0012) for the placebo arm. However, for the CPS ≥1 group, mPFS was only 7.6 months with the addition of pembrolizumab versus 5.6 months without, which did not meet statistical significance. Due to the hierarchical multiplicity strategy that was implemented, statistical significance was not formally tested in the ITT group, but mPFS was found to be 7.5 months versus 5.6 months, respectively.
Secondary outcomes were later presented as an abstract at the 2020 Virtual San Antonio Breast Cancer Symposium (SABCS).58 When analysing the ITT cohort, the ORR was 41.0% (versus 35.9% for placebo), the disease control rate (DCR) was 56.0% (versus 51.6%), and the median duration of response (mDOR) was 10.1 months (versus 6.4 months). All three outcomes further improved with increased PD-L1 expression, as seen in the CPS ≥10 group, with an ORR of 53.2% (versus 39.8% for placebo), DCR of 65.0% (versus 54.5%) and mDOR of 19.3 months (versus 7.3 months). The abstract also included individual PFS data for each chemotherapy agent used, though the trial was underpowered for comparing efficacy among the individual regimens. Chemotherapy regimens ranked from highest to lowest mPFS in the ITT population were: paclitaxel (8.0 months versus 3.8 months for the control, HR 0.57, 95% CI 0.35–0.93), nab-paclitaxel (7.5 versus 5.4 months, HR 0.69, 95% CI 0.51–0.93) and gemcitabine/carboplatin (7.4 versus 7.4 months, HR 0.93, 95% CI 0.74–1.16). This pattern remained the same for the CPS ≥1 and CPS ≥10 groups, even as the gap in mPFS widened between the experimental and control arms as CPS increased.
In summary, KEYNOTE-355 showed that there was a statistically meaningful benefit in PFS for patients with a CPS ≥10. Given these findings, the FDA issued an accelerated approval of pembrolizumab plus chemotherapy for use as first-line therapy in patients with PD-L1-postitive (CPS ≥10) mTNBC.18 In July 2021, the US FDA granted regular approval to pembrolizumab plus chemotherapy based on the final survival analysis, which demonstrated improved median survival (23 verses 16.1 months for the control (HR 0.73, 95% CI 0.55 – 0.93).59 The National Comprehensive Cancer Network (NCCN) has also made a category 1 recommendation regarding the combination of pembrolizumab with the chemotherapy options listed above in patients with tumours that have a CPS ≥10 based on the Dako 22C3 IHC assay.60
Discussion
There has been a wave of new immunotherapy trials involving mTNBC in recent years. The individual designs and results of these trials are conveniently summarized for easy comparison in Table 2.29,33,49–52, 54–56,58,59
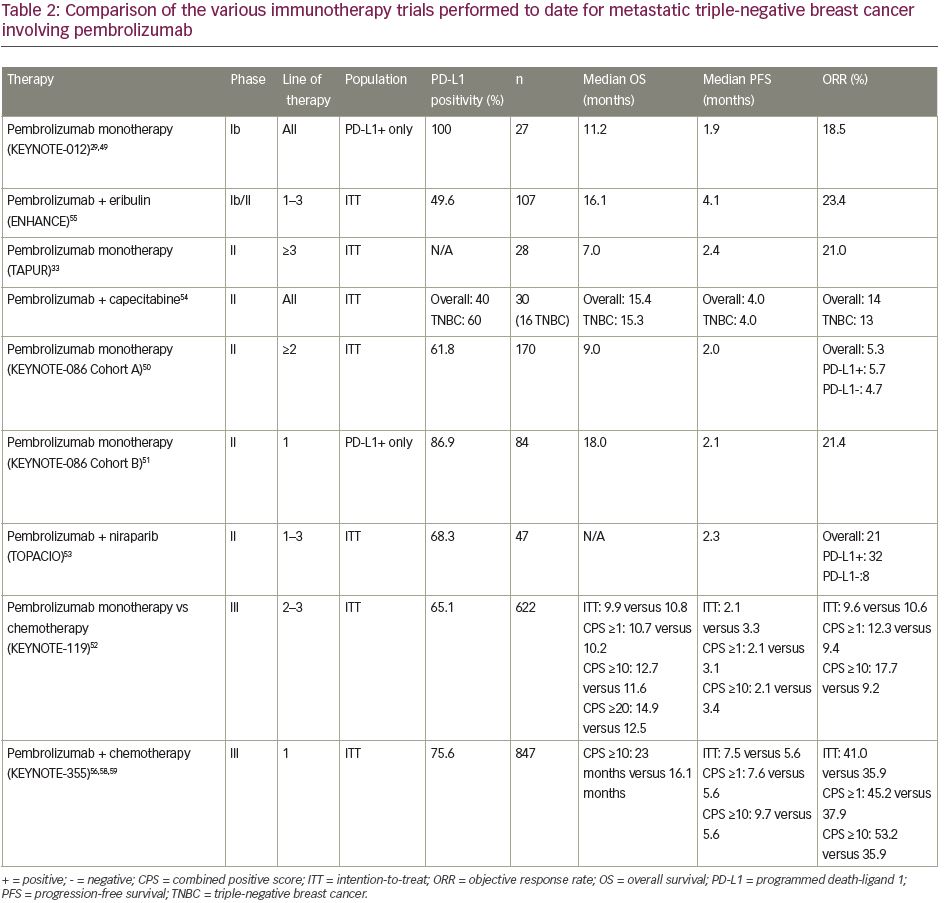
When reviewing the summarized results of monotherapy trials, it becomes clear that outcomes are consistently inferior to combination regimens, limiting their utility as stand-alone treatments, in contrast to other cancer types. This is true even when evaluating alternative immunotherapy agents, including atezolizumab and avelumab. The most notable trial regarding ICI monotherapy is KEYNOTE-119. Given that this direct comparison study of immunotherapy versus chemotherapy omitted platinum and taxane regimens (some of the most active agents in TNBC), it is hard to argue for the use of ICI monotherapy in place of conventional chemotherapy until those options have been explored first. The place where ICI monotherapy may eventually play a role, as seen in the post hoc analysis of KEYNOTE-119, is in mTNBC that expresses PD-L1 at exceptionally high levels, though this represents lower quality data that needs further vetting.61 Similar to non-small cell lung cancer (NSCLC), we may find that beyond a certain cut-off (e.g. CPS ≥50), ICI monotherapy might suffice in comparison with combination therapy, with the understanding that this would represent a slim minority of the total mTNBC population. Monotherapy could also be considered in patients with MSI-H or TMB-H disease, though again this occurs infrequently.
For combination therapy, results have been more promising, though equally complex. One of the earliest phase III breast cancer trials to implement ICI therapy for the treatment of mTNBC was IMpassion130, which was a randomized placebo-controlled trial (n=902) that enrolled patients with untreated metastatic or locally advanced TNBC who received nab-paclitaxel +/- atezolizumab.62,63 The trial demonstrated a numerical but statistically insignificant increase in mOS in both the ITT and PD-L1 positive (defined as SP142 immune cells ≥1%) cohorts with the addition of atezolizumab (21.0 months versus 18.7 months and 25.4 months versus 17.9 months, respectively). However, compared with nab-paclitaxel alone, adding atezolizumab significantly improved mPFS both in the ITT group (7.2 months versus 5.5 months; p=0.002) and in the PD-L1-positive subgroup (7.5 months versus 5.3 months; p<0.001). Following the success of IMpassion130, atezolizumab/nab-paclitaxel was streamlined to first-line therapy for the treatment of mTNBC, and implemented into the NCCN and ESMO guidelines, specifically for use in patients with PD-L1-positive mTNBC.60,64
Interestingly, IMpassion131 evaluated paclitaxel +/- atezolizumab as first-line therapy in patients with PD-L1-positive mTNBC. However, it did not demonstrate an improvement in PFS between the two groups.65 In September 2020, the FDA issued an efficacy and safety alert based on the trial results.66 Approximately one year later, on August 27, 2021, the manufacturer of atezolizumab (Genentech) voluntarily withdrew the accelerated approval indication for atezolizumab plus nab-paclitaxel in mTNBC. Continued approval of atezolizumab in the mTNBC setting was contingent upon the final IMpassion131 study results, of which were published in early August 2021 and showed that the study failed to meet its endpoint.67
When we compare both IMpassion studies to KEYNOTE-355, we find the designs were quite similar. Patient populations and characteristics were alike among these studies, including the percentage of patients who had been previously exposed to taxanes in the neoadjuvant or adjuvant settings for non-metastatic disease. ORR was lower in KEYNOTE-355, both for the ITT (41.0% versus 56.0% and 54.0%) and PD-L1-positive (45.2% versus 58.9% and 63.0%) groups compared with the IMpassion130 and IMpassion131, respectively. However, with an ITT mPFS of 7.5 months (versus 7.2 and 5.6 months in IMpassion130 and IMpassion131, respectively) and a PD-L1-positive subgroup mPFS of 7.6 months (versus 7.5 and 5.7 months), it becomes clear that, as with most ICI therapies, duration of response plays a stronger role than initial response rates. This brings up an ongoing question regarding what factors impair treatment response. Data regarding immune-related adverse events (irAEs) were limited in the IMpassion studies, but for KEYNOTE-355, grade 3 irAEs were reported in 5% of the pembrolizumab arm versus 0.0% in the placebo arm, with rash being the most common event (2.0% total). For all grades of irAEs, 26.0% were reported for the experimental arm versus 6.0% in the placebo arm, most commonly thyroid disease (20.0% versus 4.0%).
One of the initial concerns regarding the use of ICI therapy in breast cancer revolved around its intrinsically low expression of PD-L1. Surrounding immune cells make up the majority of positive PD-L1-expressing cells when using both PD-L1 assays, whereas less than 25.0% of the malignant breast cells themselves express PD-L1 on their surface.42 Contrast this with more immunosensitive tumours, such as NSCLC, where surface PD-L1 expression on malignant cells and immune cells is near equal.68–70
Conclusion and future directions
There is mounting evidence regarding the benefit of immunotherapy in breast cancer. A clear clinical benefit is observed when immunotherapy is combined with conventional chemotherapy as first-line treatment for mTNBC that expresses PD-L1, particularly cancers with higher expression, such as those with a CPS ≥10.
Future studies involving ICIs in breast cancer include those that combine ICIs with various treatments, such as radiation therapy, PARP inhibitors, HER2-directed therapy, cyclin-dependent kinase (CDK) 4/6 inhibitors, mitogen-activated protein kinase kinase (MEK) inhibitors, epigenetic therapy, and indoleamine-pyrrole 2,3-dioxygenase (IDO) inhibitors.71 The number of novel immune checkpoint agents is also on the rise, with many already entering phase I/II trials, usually in the form of combination therapy.72,73 These include drugs that target inhibitory immune checkpoints (LAG3, TIM-3, TIGIT, VISTA, B7-H3) along with co-stimulants (OX-40, GITR, CD27, CD137). Some are even engineered as viral vectors (e.g. adenovirus vector-mediated OX40 ligand gene transfer) or bispecific antibodies (e.g. MGD013, PD-1 and LAG3) to further improve efficacy.74 Given this explosion of new-wave pharmaceuticals being tested, the standard of care for mTNBC will continue to evolve in years to come with the hope of improving clinical outcomes in patients with breast cancer.