While the incidence of primary breast cancer has increased recently, most patients are diagnosed with early-stage disease and good prognosis. Around 80 % of patients with early breast cancer have estrogen receptor (ER)-positive disease and their prognosis will be improved substantially by endocrine therapy.1 Nevertheless, the rate of distant recurrence (DR) in these patients can be as high as >20 % in the first 10 years and this rate can be reduced by adjuvant chemotherapy.2
It is a priority in clinical breast cancer practice to differentiate patients at high risk of recurrence (ROR) from the majority at low risk (i.e. ≤10 % over 10 years) who may be spared chemotherapy.3 The diversity in breast cancer, in terms of molecular alterations, cellular composition and clinical outcome, creates a challenge for developing tumour classifications that are clinically useful for prognosis estimation.4 The heterogeneity of breast cancer can be categorised into distinct subgroups called ‘intrinsic’ subtypes that are defined by their pattern of gene expression and clinical outcome: Luminal A, Luminal B, human epidermal growth factor 2 (HER2)-enriched and basal-like.5,6 These intrinsic subtypes have been studied extensively,6 9 and the St Gallen International Expert Consensus has recognised that these intrinsic subtypes provide key information for treatment decision-making in early breast cancer.10,11
The intrinsic subtypes were first identified and long studied using genome-wide RNA expression profiling or DNA microarrays, and fresh frozen tissue. In order to identify the original intrinsic subtypes using the clinically relevant formalin-fixed paraffin-embedded (FFPE)
tumour specimens, a 50-gene signature was first constructed using the Prediction Analysis of Microarray (PAM) method. More than 93 % accuracy in subtype classification was demonstrated using the PAM50 versus the original gene classifier consisting of about 1,900 genes12 and the PAM50 signature has since been transferred to the NanoString nCounter technology for use as a clinical diagnostic.13
Prosigna is the only genomic test to identify the individual tumour’s intrinsic subtype according to the PAM50 signature. In addition to identification of the intrinsic subtype, Prosigna also provides an individualised score, the ROR, which estimates a patient’s probability of DR by weighting the intrinsic subtype correlations, a subset of proliferation genes and pathological tumour size as well as nodal status.4,14 The PAM50 intrinsic subtypes and the ROR classifier have been integral to the development of the Prosigna Breast Cancer Prognostic Gene Signature Assay, a US Food and Drug Administration (FDA) 510(k) cleared in vitro diagnostic assay that uses the gene-expression profile of the breast cancer cells to assess a patient’s risk of DR.
Prosigna Breast Cancer Prognostic Gene Signature Assay
The Prosigna assay measures a gene-expression profile using RNA extracted from FFPE breast tumour tissue. The gene-expression data are weighted together with clinicopathological variables (i.e., tumour size and number of positive nodes) to generate an intrinsic subtype and a score indicative of the probability of distant disease recurrence. The assay has been validated on the NanoString nCounter Analysis System.13
After the assay is performed on a tumour sample, a computational algorithm based on Pearson’s correlation compares the normalised 50 gene-expression profile of the patient sample to the prototypical expression profile of the intrinsic subtypes. The tumour sample is then assigned the subtype that shows the highest Pearson’s correlation. The algorithm also reports a ROR score on a 0–100 scale.15 This ROR score is correlated with the probability of DR at 10 years for postmenopausal women with ER-positive, early-stage breast cancer.14 The report provides a risk category (low, intermediate or high), which is based on the ROR and derived from the probability risk curves. ROR has been shown to discriminate between high- and low-risk groups in node-negative and node-positive tamoxifen-treated patients.14 Prosigna ROR and risk assignment takes into account patient nodal status. As nodal status is an independent risk factor, the node-positive population has a risk curve that is shifted towards higher risk compared with the node-negative population. Therefore, to maintain similar outcomes for the risk groups between node-negative and node-positive disease, the cut-off points used for risk assignment differ between node-negative and node-positive patients.
Analytical Validation
The performance of the Prosigna Breast Cancer Prognostic Gene Signature Assay was validated on the nCounter Analysis System, across three laboratories for reproducibility, precision and robustness.13 RNA input range was validated by comparing assay results at the extremes of the specified range to the nominal RNA input level.13
Precision Assessment
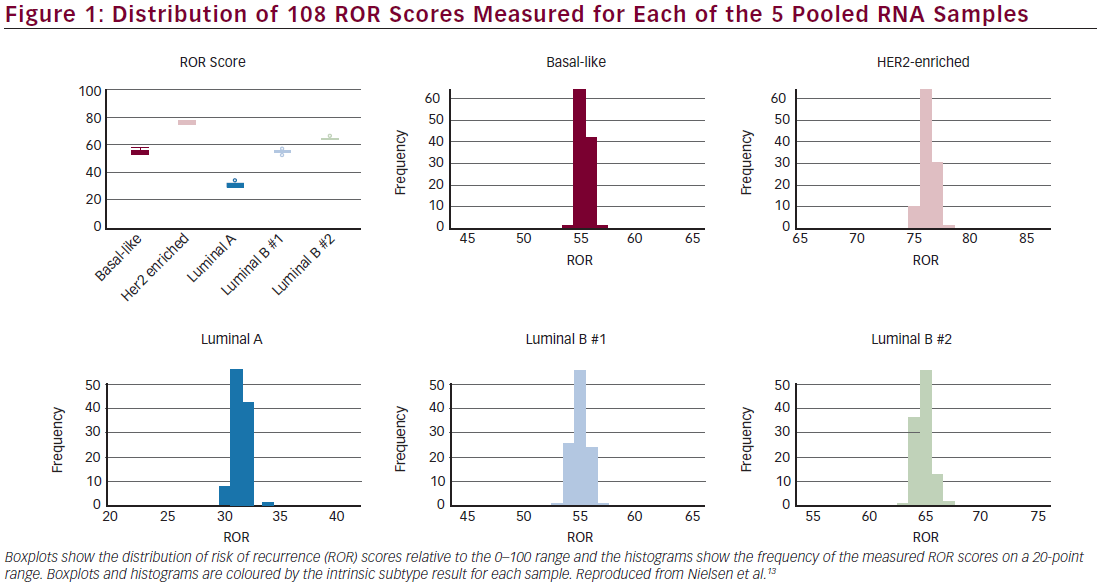
Precision was derived from 108 replicates of each of five pooled RNA samples, at three different sites, two operators at each site, each with three different reagent lots. There was 100 % concordance between the measured subtype result and expected subtype result and 100 % concordance between measured and expected risk category.13,16 The mean ROR difference between reagent lots on a 0–100 scale was <1 ROR unit apart across all 108 measurements for each of the five pooled breast tumour RNA samples (see Figure 1).16 The variance components analysis resulted in a total standard deviation of 0.67.16 There was no detectable site-to-site or operator-to-operator variance (<1 % of total variance), a result that could be critical for a de-centralised test.16
Reproducibility Assessment
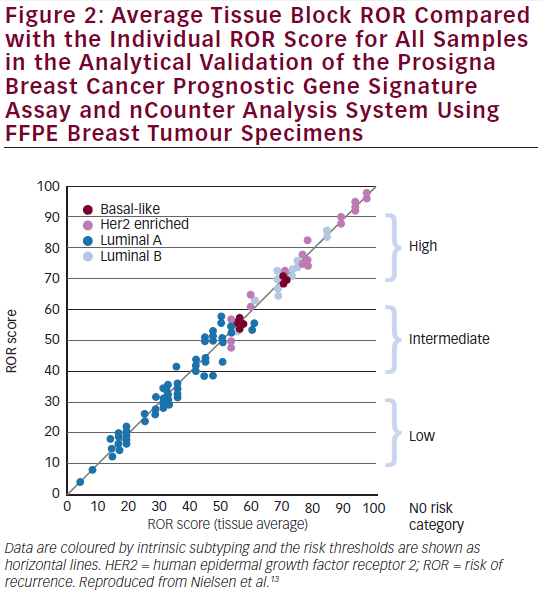
Following an independent pathology review at each site, the reproducibility was assessed by testing replicate tissue sections from 43 FFPE breast tumour blocks across the three sites and nCounter platforms.13,16 The calculated test results from the 43 tissues across all sites represented (see Figure 2) a wide range of ROR scores (94 units); all risk categories; and all four intrinsic subtypes.13,16 On average, the differences between the sites were minimal (<1 % of total variance). The variance components analysis resulted in a total standard deviation of 2.89 ROR units. The average concordances between sites were: 97 % for intrinsic subtype classifications and 90 % for risk classifications.
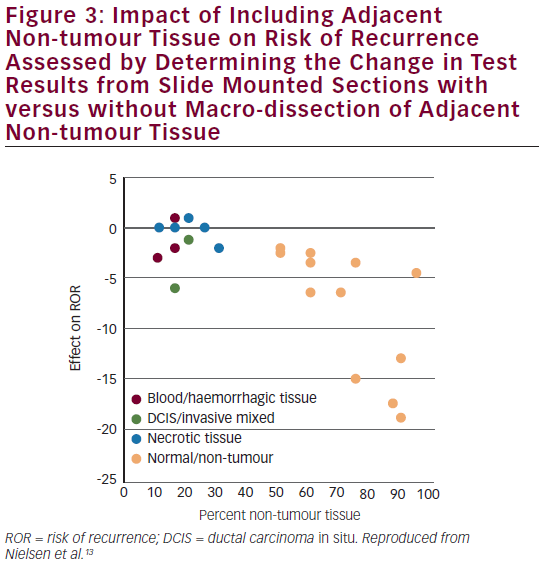
Assessment of Robustness Non-tumour tissue was included in the test to evaluate interference and the robustness of the test to non-tumour tissue.13 The Prosigna test was robust against the inclusion of adjacent non-tumour tissue in the assay. Results were stable in the presence of moderate amounts of surrounding non-tumour tissue (maximum 70 % by area) (see Figure 3). Results were also shown to be stable across the specified range of RNA input.
These data demonstrate that the Prosigna assay is analytically reproducible, precise and robust, even when performed at multiple test sites and including all potential process variables. Together with the clinical validation, this supporting evidence for a decentralised test led to FDA clearance (510K) in September 2013. This differentiates the newgeneration Prosigna assay from other first-generation tests that are performed in a central laboratory. If an nCounter system is available at a site, the decentralised test enables pathologists to perform the assay locally that allows for direct access for patients and a fast turnaround time of results.
Clinical Validation
Prosigna has been clinically validated by prospectively defined analysis of two registration-quality databases, the Arimidex, Tamoxifen Alone or Combined (ATAC) trial (NCT00849030)15 and the Austrian Breast & Colorectal Cancer Study Group 8 (ABCSG 8) trial (NCT00291759)17 using a total of >2,400 patient samples. Both studies had ≥10-year median follow-up in post-menopausal women with ER-positive early-stage breast cancer treated with endocrine therapy alone. The primary objective was to validate published observations that Prosigna ROR score provides
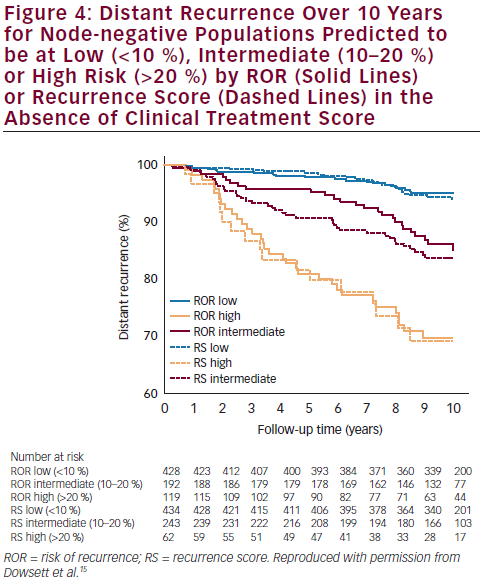
additional prognostic information over and above standard clinical variables for DR-free survival at 10 years. In the TransATAC study, mRNA from 1,017 patients with ER-positive primary breast cancer treated with anastrozole or tamoxifen in the ATAC trial was assessed to determine the intrinsic subtype and ROR using the nCounter technology. The Prosigna ROR score was shown to provide clinically relevant prognostic information in endocrine-treated patients with ER-positive, node-negative disease.15 ROR provided prognostic information beyond the clinical treatment score (CTS) in all patients (Δ LR-χ2 = 33.9; p<0.001) and in all four subgroups: node-
negative, node-positive, HER2-negative and HER2-negative/nodenegative; more information was added by ROR than by Oncotype Dx® recurrence score (RS). Concordance indices in the HER2-negative/ node-negative subgroup were 0.670, and 0.769 for RS, and ROR, respectively. Furthermore, ROR displayed improved differentiation of intermediate- and higher-risk groups compared with RS. More patients were scored as high risk and fewer as intermediate risk by ROR than by RS (see Figure 4).
As treatment decisions are the most difficult for patients classified as intermediate, precise assignment of patients to risk groups is needed. In a meta-analysis of the impact of Oncotype Dx breast cancer assay in clinical practice, nearly 40 % of patients were assigned to an intermediate risk category classification where optimal treatment is unclear.18 Ongoing prospective trials such as TAILORx (NCT00310180), RxPONDER (NCT01272037), WSG-PlanB (NCT01049425) and WSG-ADAPT19 (ADAPT Umbrella: NCT01781338; ADAPT HER2+/HR+:NCT01745965; ADAPT HER2+/HR-: NCT01817452; ADAPT TN: NCT01815242) are evaluating actual risk and optimal therapy for these intermediate-risk patients. Determining the intrinsic subtype, especially in the intermediate risk group, may also be helpful to guide clinical decision-making.20
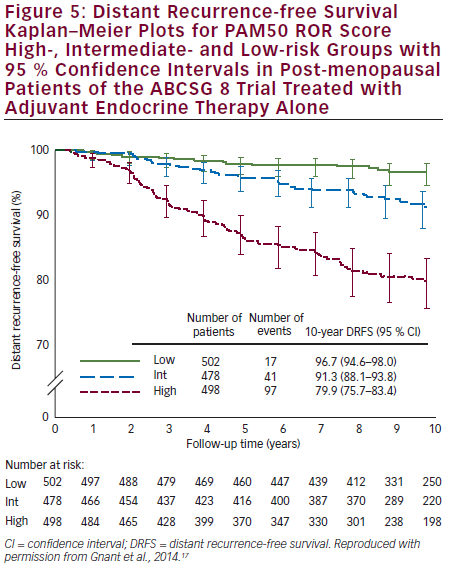
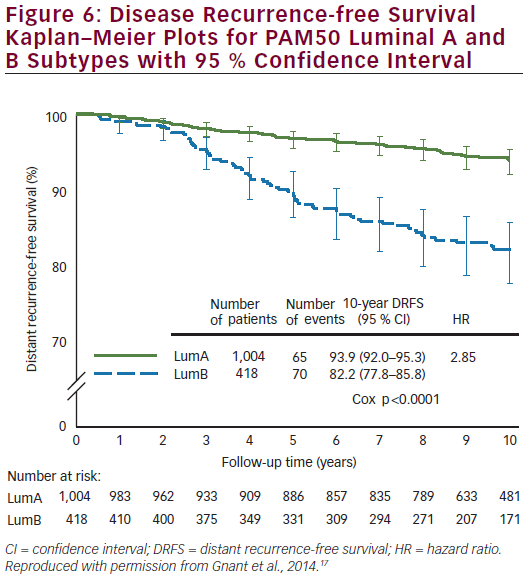
Prosigna was further validated as a reliable prognostic indicator for DR-free survival (DRFS) in hormone receptor-positive, post-menopausal patients from the ABCSG 8 trial.17 ABCSG 8 was first designed to evaluate the clinical efficacy of tamoxifen versus anastrozole after 2 years of tamoxifen in >3,700 patients. A translational study of the ABCSG 8 was conducted with 1,478 FFPE blocks available. ABCSG 8 patients were postmenopausal women treated with adjuvant systemic endocrine therapy alone in the form of 5 years of tamoxifen or 2 years of tamoxifen and 3 years of anastrozole. Prosigna ROR scores significantly added prognostic value to the clinical predictor (p<0.0001) in all tested subgroups. Within all tested subgroups, significant and clinically relevant discrimination was demonstrated between low- and high-risk groups (see Figure 5). The Luminal A intrinsic subtype cohort had a significantly lower ROR at 10 years compared with Luminal B tumours (p<0.0001) (see Figure 6). The 10-year metastasis risk in the ROR low-risk category for all patients combined (i.e. node-positive and node-negative) was <3.5 %.
The results of this analysis, which are concordant with the results of the analysis from the ATAC trial,15 constitute Level 1B evidence for clinical validity of the PAM50 test for predicting the risk of DR in post-menopausal women with ER-positive early breast cancer (see Table 1). The absolute risk of DR of <3.5 % by year 10 in the low-risk population means that adjuvant chemotherapy would have approximately 1 % absolute benefit, so such patients could be spared unwarranted over-treatment that could result in a similar risk of serious adverse events.10 The test also identified a low-risk subgroup within node-positive patients. There is a clear difference in outcomes for Luminal A compared with Luminal B subtypes, which may also be valuable clinical information.10
More recently, combined analysis of the two pivotal trials, ABCSG 8 and ATAC, demonstrated that the PAM50-derived ROR, ROR risk groups and intrinsic subtypes provide reliable prognostic information for metastasis risk in addition to and beyond established clinicopathological factors in node-positive hormone receptor-positive breast cancer.21 In this large dataset (543 node-positive patients), low- and high-risk patients were accurately discerned. RNA from paraffin blocks was analysed using the PAM50 test and the primary objective was to investigate the prognostic information provided by the ROR score added to combined clinical standard variables, using log-likelihood tests. At a median follow-up of 6.6 % years, distant metastases occurred in 97 (18 %) of the 543 nodepositive patients. The 10-year DR risk was increased significantly in the high-risk group compared with the low-risk group derived from ROR score for one-positive node (25.5 %, 95 % confidence interval [CI] 17.5–36.1 % versus 6.6 %, 95 % CI 3.3 %–12.8 %) and compared with the combined low/intermediate risk group for patients with two or three positive nodes (33.7 %, 95 % CI 25.5–43.8 % versus 12.5 %, 95 % CI 6.6–22.8 %). Further, the Luminal A intrinsic subtype was associated with significantly lower
risk of DR compared with the Luminal B subtype in patients with one positive node and in patients with two or three positive nodes.
Late Recurrence for Estrogen Receptorpositive Breast Cancer
The prediction and prevention of late breast cancer recurrence is an important and largely unmet need and remains a major clinical challenge. Indeed, despite recent improvements in breast cancer treatment, some women with ER-positive early-stage breast cancer remain at risk of DR beyond the first 5 years following diagnosis.1 Multiple clinical trials have shown that the risk of late DR may be reduced by extending adjuvant endocrine therapy beyond 5 years.22–24 Identifying newly diagnosed women with ER-positive breast cancer at high risk of having their cancer recur between 5 and 10 years after diagnosis is a priority to help oncologists to make more informed treatment decisions.25
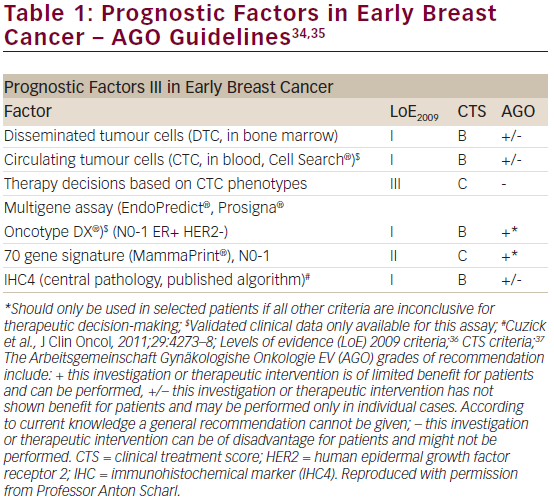
Adjuvant chemotherapy and endocrine therapy for early breast cancer have had a positive impact on outcomes;1 however, the annual rate of late recurrence is still >2 % for ≥15 years, even after 5 years of tamoxifen therapy.26 The value of three breast cancer assay scores in predicting the risk of DR has been assessed in years 5 to 10:27 the PAM50 gene signature, the Oncotype Dx® Breast Cancer Assay RS and the immunohistochemical marker (IHC4) score, were included. The IHC4 score, which may be subject to large inter- and intra-observer variability,28 was originally derived from a centralised IHC assessment of ERs, progesterone receptors (PR), HER2 and Ki67 expression in the TransATAC cohort.
A total of 940 samples from the TransATAC dataset were included in this study. RS and ROR were assessed from RNA extracted from tumour samples by Genomic Health, Inc. for validation of the Oncotype Dx® Breast Cancer Assay. Univariate and multivariable proportional hazards models were used and statistical tests were all two-sided. In multivariate analyses including all patients, the IHC4, RS and ROR scores all added statistically significant prognostic information in years 5 to 10. ROR score was shown to be the strongest prognostic factor in the late follow-up period (χ²=16.29; p<0.001). IHC4 (χ²=7.41) and RS (χ²=5.55) were only weakly prognostic in the late follow-up period. In a multivariate analysis of the clinically relevant subgroup of HER2-negative, node-negative patients the Prosigna ROR score remained a statistically significant predictor of
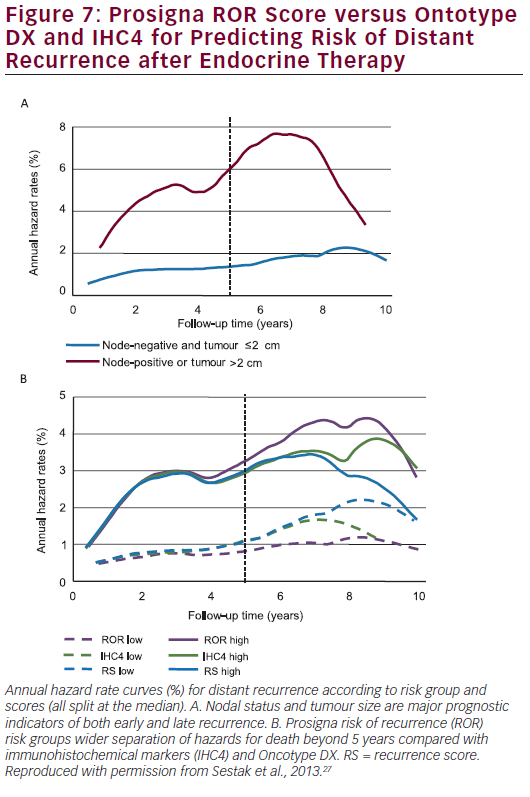
late DR (χ²=13.85; p<0.001) while IHC4 was marginally significant (χ²=3.89; p=0.05) and RS was not significant (χ²=2.23; p=0.1). The difference in DR rate between the low- and high-risk groups is approximately 7 % for all scores in years 0 to 5. However, in years 5 to 10, a greater difference between low- and high-risk groups is seen for the ROR score (15.1 %) compared with 5.4 % for RS and 9.8 % for the IHC4 score. Figure 7A shows annual hazard rates for low-risk women (node-negative and tumour size ≤2 cm) versus high-risk women (node-positive or tumour size >2 cm) whereas Figure 7B shows annual hazard rate curves for IHC4, RS and ROR (cut-off point for all scores is the median).
The PAM50 ROR score and ROR‐based risk groups have also been shown to provide significant additional prognostic information in terms of late DRFS compared with a combined score of clinical factors alone in the ABCSG 8 study.29 Between years 5 and 15, the observed absolute risk of DR was 2.4 % in the low ROR‐based risk group compared with 17.5 % in the high ROR‐based risk group. The DRFS differences according to the PAM50 ROR score were observed for both node‐positive and nodenegative disease. These results indicate that the PAM50 ROR score and ROR‐based risk groups can differentiate patients with breast cancer with respect to their risk for late DR beyond what can be achieved with established clinicopathological factors.29
The ROR score may thus also be useful in identifying women who are at high risk of late recurrence and who may benefit from either more intensive treatment (chemotherapy) or extended endocrine treatment, beyond 5 years.

Local Recurrence for Estrogen Receptorpositive Breast Cancer
The PAM50 ROR score and intrinsic subtype have been demonstrated to be independent predictors of local recurrence-free survival (LRFS).30 Tumour blocks from 1,308 patients from the ABCSG 8 trial comparing tamoxifen versus tamoxifen following anastrozole post-surgery were analysed by the PAM50 assay. The Cox regression model revealed that the ROR score significantly predicted LRFS independent of nodal status, tumour size and age (p<0.0081). Patients assigned to the high-risk category (ROR ≥57) had a LRFS of 94.4 % whereas low-risk patients (ROR <57) showed a 98.4 % 10-year LRFS (p=0.0005). Biological subtype analysis also showed a significant difference in LRFS comparing Luminal A (98.1 % 10-year LRFS) and Luminal B cancer (95.9 % 10-year LRFS; p=0.022).
Decision Impact Studies
There are three European decision impact (DI) studies: one (France; multicentre) in progress and two (Spain; GEICAM group;31 Germany; West German Study Group32) that have already been completed.
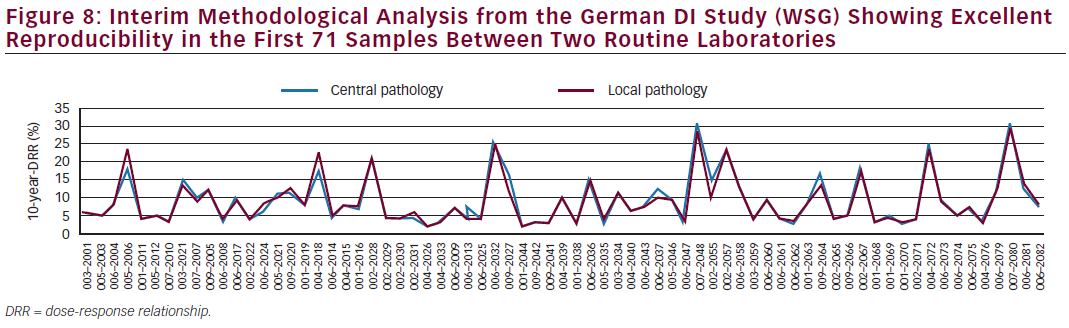
The three studies aimed at recruiting 200 patients each and the protocols are identical. The aim is to examine whether the Prosigna test influences physician and patient adjuvant treatment selection beyond standard local IHC testing. A meta-analysis of the three studies is planned to yield a larger DI database. The Spanish GEICAM decision impact study showed that Prosigna indeed influenced adjuvanttherapy recommendations.31 Prosigna changed treatments in 20 % of patients. For 22 (11 %), the initial recommendation was changed from chemotherapy and hormonal therapy to hormonal therapy alone and for 18 (9 %), from hormonal therapy to chemotherapy and hormonal therapy. Further, Prosigna increased physician confidence in prognosis and in recommended treatments while patient anxiety was reduced. Initial results from the German DI Study indicate rather similar results with a change in treatment choice of 18.2 % after obtaining the Prosigna results.32 Final results of the German DI study will be reported by the end of 2015. An interim quality assurance analysis from the German DI study showing excellent reproducibility in the first 71 samples between two routine laboratories is depicted in Figure 8.
Conclusions
The Prosigna assay is based on the PAM50 gene signature. It indicated for post-menopausal women with early-stage ER-positive/Her2-negative breast cancer with or without axillary lymph node involvement (main features are summarised in Table 2). It has been demonstrated to be analytically reproducible, precise and robust and has been independently clinically validated. The Prosigna assay was approved by the majority of the panel at the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015 for its prognostic strength for early (years 1–5) as well as late (years 5–10) recurrences.33
In a prospectively planned retrospective comparison of the Prosigna, the Oncotype Dx Breast Cancer Assay and the IHC4 score, the Prosigna ROR score provided the strongest prognostic information regarding risk of DR in 5 to 10 years following diagnosis in post-menopausal ER-positive early-stage breast cancer patients treated with 5 years of endocrine therapy.27 Separate analysis of the PAM50 ROR score for prediction of DR and late DR (beyond 5 years after diagnosis and treatment) in 1,478 post‐menopausal patients from the ABCSG 8 trial also validated the test for these endpoints after a median follow up of 11 years.29
The Prosigna ROR score differentiates patients with post-menopausal, endocrine responsive breast cancer in terms of their risk of late DR, and physicians can stratify patients into overall risk categories with different prognoses. The Prosigna assay can also be used to assign intrinsic subtypes to all cases and DRFS is significantly different between Luminal A and Luminal B subtypes. In the combined analysis, 15-year late DRFS was 92.8 % for the Luminal A and 86.2 % for the Luminal B group. The prognostic differences between Luminal A and Luminal B intrinsic subtypes were less pronounced compared with that between the ROR low- and high-risk groups.29
The Prosigna ROR also identifies patients at high risk of local recurrence.30 Last but not least, DI evidence has provided additional data showing that the Prosigna ROR assay has clinical utility in the real-world setting. Its application seems to be associated with at least a 20 % change in treatment decisions. Additional ongoing DI studies will provide realworld data in various healthcare settings with regard to analytical validity, change of treatment decisions as well as health economic analyses. In view of its broad database in terms of prognostic estimation and intrinsic subtyping in post-menopausal patients, an open clinical question that needs to be addressed for the Prosigna assay is certainly its validation for pre-menopausal patients.
Overall, the Prosigna assay may enable clinicians to make more informed treatment choices for women with endocrine responsive breast cancer by determining the intrinsic subtype as well as shortand long-term ROR. This may help to help identify women who may be spared adjuvant chemotherapy or who may benefit from extended adjuvant endocrine therapy.













