Globally, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in women.1 More than 1.67 million cases of breast cancer were recorded in 2012, accounting for around 11.9% of all new cancer cases and 25.1% in women.2 Oestrogen receptor- (ER)-positive breast cancer accounts for 75% of breast cancers in postmenopausal women.3 Aromatase inhibitors (AIs) and anti-oestrogens are used in the adjuvant endocrine therapy of postmenopausal women with ER-positive breast cancer. AIs constitute the standard of care in the first-line management of patients with hormone-receptor positive breast cancer.4 Both anastrozole and letrozole have proven superior to tamoxifen as five years’ primary adjuvant therapy in early breast cancer, in addition to providing a number of tolerability benefits compared with the tamoxifen.5 The selective oestrogen receptor modulator (SERM) tamoxifen is also used widely to treat both premenopausal and postmenopausal patients with advanced breast cancer as firstline treatment.6 Other endocrine therapies include the progestins, such as megestrol acetate, high-dose oestrogens and androgens. However, these treatment options are being used less frequently as newer, more effective and better-tolerated therapies become available. Although adjuvant endocrine therapy is an effective treatment for breast cancer, most patients with advanced disease will eventually exhibit resistance to individual therapies. Nonetheless, an initial response to endocrine treatment is generally indicative of a positive response to further alternative endocrine agents.7
Selective oestrogen receptor downreglators (SERDs) is a novel class of anti-oestrogens that differ from SERMs in being a full or ‘pure’ receptor antagonist. Fulvestrant is a SERD that competitively binds to oestrogen receptors (ER), with approximately 100-times greater binding affinity than that of tamoxifen.8 Following binding to ER, fulvestrant blocks dimerisation of the receptor and limits its nuclear translocation. The fulvestrant-bound ER complex is unstable and more susceptible to degradation, resulting in downregulation of receptor expression. Fulvestrant also blocks the recruitment of both transcriptional activating factors (AF-1 and AF-2) to ER, which are needed for full activation of the transcription of ERregulated genes. In contrast, tamoxifen blocks recruitment of AF-2 only. Hence, fulvestrant exhibits full ER antagonist activity, while showing no known oestrogen agonist activity.9It does not show cross-resistance with tamoxifen, or the oestrogen receptor agonist activity associated with tamoxifen. Fulvestrant has been shown to be active in patients with breast cancer previously treated with a SERM such as tamoxifen or with a non-steroidal anti-inflammatory (AI) such as anastrozole.10
Effect of fulvestrant on tumour receptors Robertson et al. conducted a randomised study in postmenopausal women with primary breast cancer (T1–T3, ER+ or ER status unknown). Fulvestrant was administered as a single intramuscular (IM) dose of either 50 mg (n=39), 125 mg (n=38) or 250 mg (n=44), 14–21 days prior to tumour resection, or continuous oral tamoxifen (20 mg daily; n=36)or matching tamoxifen placebo (n=43). Tumour biopsies taken prerandomisation and at surgery were compared.11
• ER downregulation: fulvestrant reduced the ER index in a dosedependent manner. The ER index was suppressed significantly by each of the three doses of fulvestrant (50 mg, 125 mg and 250 mg) when compared with placebo and by the highest dose of fulvestrant (250 mg) when compared with tamoxifen (p=0.024).
• Progesterone receptor (PgR) downreglation: a statistically significant and dose-dependent reduction in the PgR index was demonstrated for all doses of fulvestrant, whereas PgR levels increased after tamoxifen therapy, consistent with its partial agonist effects and the presence of a functional ER pathway. The decrease in the PgR index for the 125 mg and 250 mg doses of fulvestrant was also statistically significant, when compared with placebo.
• Ki67 downreglation: fulvestrant produced a dose-dependent reduction in Ki67 labelling. All three doses of fulvestrant significantly reduced the Ki67 index when compared with placebo and, to a similar extent, that seen with tamoxifen.11
Dose-dependent effect of fulvestrant on receptors
Kuter et al. conducted a randomised, open-label, phase II study in postmenopausal women with ER positive locally advanced breast cancer to compare fulvestrant 500 mg (500 mg/month plus 500 mg on day 14 of month 1) with fulvestrant 250 mg/month for 16 weeks prior to surgery. Core biopsies at baseline, week 4, and at surgery were assessed for biomarker changes. A total of 211 patients were randomised (fulvestrant 500 mg: n=109; 250 mg: n=102). Fulvestrant 500 mg resulted in greater reduction of Ki67 labeling index (LI) and ER expression versus 250 mg (–78.8 versus –47.4% [p<0.0001] and –25.0 versus –13.5% [p=0.0002], respectively at week 4; PgR suppression was not significantly different (–22.7 versus –17.6; p=0.5677). Tumour response rates were 22.9% and 20.6% at week 16 for fulvestrant 500 mg and 250 mg, respectively, with considerable decline in all markers. No detrimental effects on endometrial thickness or bone markers and no new safety concerns were identified. This provided the first evidence of greater biological activity for fulvestrant 500 mg versus 250 mg in depleting ER expression, function and growth.12
Effect of fulvestrant on sex hormones, bone turnover markers and endometrial thickness
There were no clinically significant changes in serum levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH) or sex hormone-binding globulin (SHBG).13,14 On the other hand, tamoxifen is associated with significantly (p<0.001) increased SHBG and decreased LH and FSH levels from baseline.15
In a small study of 14 patients by Agrawal et al., patients with advanced breast cancer treated with fulvestrant 250 mg monthly up to 18 months were assessed for bone-specific turnover markers like serum bone-specific alkaline phosphatase (BAP), N-terminal propeptide of procollagen type 1 (PINP) and C-terminal telopeptide (CTX) at 0, 1, 6, 12 and 18 months. No clinically significant changes were observed in bone-specific turnover markers.16 Also in the NEWEST trial, clinically significant changes were not observed with either the 250 mg monthly or 500 mg regimen up to 16 weeks.17
A randomised, double-blind phase I study was conducted in healthy postmenopausal women to assess the effect of fulvestrant in the endometrium.18 Thirty women were given a single intramuscular injection of fulvestrant 250 mg and endometrial thickness was measured on day 28. Mean endometrial thickness on day 28 was significantly lower in recipients of fulvestrant than in recipients of placebo (4.20 mm versus 11.22 mm; p=0.0001), following 14 days’ treatment with ethinylestradiol. Thus, there was lack of any agonist activity of fulvestrant in the endometrium of healthy postmenopausal women.
Clinical pharmacokinetics
Absorption19
Fulvestrant 500 mg undergoes slow absorption following intramuscular injection, with a mean maximum plasma concentration (Cmax) reached in approximately five days. Steady-state concentrations are reached within approximately one month.20
Distribution19,20
Fulvestrant has an extravasclar distribution with an apparent volume of approximately 3–5 l/kg at steady state. Plasma protein binding of fulvestrant is high (99%), with low-density lipoprotein, very lowdensity lipoprotein and high-density lipoprotein being the main binding components.
Metabolism19,20
Fulvestrant is metabolised by cytochrome P450 (CYP) 3A4 and not other CYP isoenzymes. Biotransformation via oxidation, aromatic hydroxylation, conjugation with glucuronic acid and/or sulphate at the 2, 3 and 17 positions of the steroid nucleus and oxidation of the side chain sulphoxide are the most likely routes of metabolism of fulvestrant.
Excretion19,20
Approximately 90% of fulvestrant is eliminated as metabolites in the faeces, with <1% excreted in the urine. Terminal elimination half-life of fulvestrant 500 mg regimen is 50 days for the 500 mg regimen.
Alterations in special populations
Fulvestrant should be used with caution in patients with a creatinine clearance of less than 30 ml/min, however, no dosage adjustment is recommended in patients with creatinine clearance of >30 ml/min. No dose adjustments are recommended for patients with Child-Pugh A (mild) and Child-Pugh B (moderate) hepatic impairment.20
Clinical evidence
Fulvestrant monotherapy studies
Two large multicentre, randomised phase III trials comparing fulvestrant with anastrozole in second-line therapy were conducted.21
• Trial 0020, conducted in Europe, Australia and South Africa
• Trial 0021, conducted in North America
Trials were prospectively designed to allow analysis of combined data. A total of 851 postmenopausal women with hormone-receptor positive advanced breast carcinoma who previously had disease progression after receiving endocrine treatment were included in the trials. The participants were randomised to receive fulvestrant 250 mg as a once-monthly (one x 5 ml) intramuscular injection or oral dose of anastrozole 1 mg daily.
There was no statistically significant difference in the time to progression (TTP) (5.5 months versus 4.1 months, respectively; hazard ratio [HR]=0.95; p=0.48) and the overall response rate (ORR) (19.2%
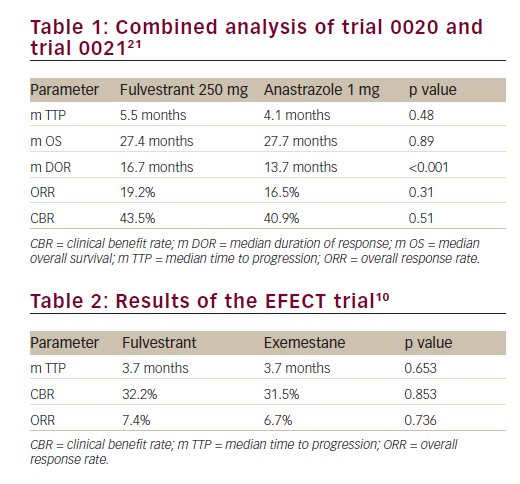
and 16.5% respectively; p=0.31) between the fulvestrant arm and anastrozole arm. The median duration of response from randomisation to disease progression was significantly higher with fulvestrant (16.7 months versus 13.7; p<0.001). The median overall survival (OS) at an extended median follow-up of 27 months were similar (27.4 months versus 27.7 months, respectively; p=0.809) (Table 1). Fulvestrant 250 mg thus demonstrated non-inferiority to the conventionally used anastrazole and was registered as an additional therapeutic option for postmenopausal patients with hormone-sensitive advanced breast cancer that have progressed on prior endocrine therapy.In a retrospective combined analysis of the two trials, there were no significant differences in the objective response and clinical benefit rates between the fulvestrant and anastrozole groups in subgroups of patients with visceral or non-visceral metastases.22 A durable median duration of response was maintained with the fulvestrant and anastrozole treatment regimens in patients with visceral (17.5 versus 11.7 months) and non-visceral (14.3 versus 13.7 months) metastases.
Chia S et al. conducted a multicenter phase III trial (Evaluation of Faslodex versus Exemestane Clinical Trial [EFECT]) in 693 postmenopausal women diagnosed with hormone-receptor positive advanced breast cancer (ABC) progressing or recurring after non-steroidal AI. The participants were randomised to receive either fulvestrant 500 mg intramuscularly on day 0, 250 mg on days 14, 28 and 250 mg every 28 days thereafter (n=351) or exemestane 25 mg orally was administered once daily (n=342).10
Median TTP was 3.7 months in both groups (hazard ratio 0.963; 95% confidence interval [CI], 0.819 to 1.133; p=0.6531). The overall response rate (7.4% versus 6.7%; p=0.736) and clinical benefit rate (32.2% versus 31.5%; p=0.853) were similar between fulvestrant and exemestane, respectively (Table 2). Median duration of clinical benefit was 9.3 and 8.3 months, respectively. Both treatments were well tolerated, with no significant differences in the incidence of adverse events or quality of life. The authors concluded that EFECT demonstrated equal clinical activity for both low dose fulvestrant and exemestane in postmenopausal hormone receptor- (HR-) positive ABC after progression during treatment with a non-steroidal AI.
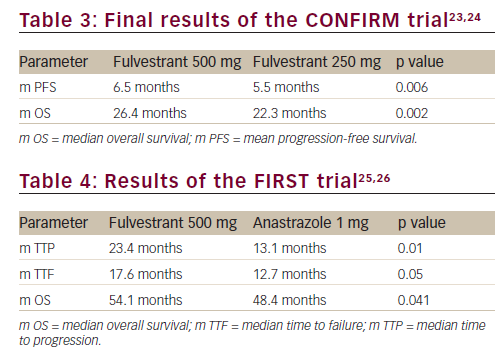
Di Leo et al. reported a double-blind, parallel-group, multicentre, phase III study named Comparison of Faslodex in Recurrent or Metastatic Breast Trial (CONFIRM) comparing fulvestrant 500 mg regimen with fulvestrant 250 mg per month for treatment of postmenopausal women with oestrogen-receptor positive advanced breast cancer who experienced progression after prior endocrine therapy. A total of 736 participants were randomised to receive either fulvestrant 250 mg regimen (250 mg on day 1, day 15 and day 29, then monthly once) or fulvestrant 500 mg regimen (500 mg on day 1, day 15 and day 29, then monthly once).23
The results demonstrated that overall response rate (p=0.795) and clinical benefit rate (p=0.100) were similar between the two groups. However, the 500 mg regimen demonstrated a significantly longer progression-free survival (PFS) than the 250 mg regimen (6.5 months versus 5.5 months, respectively; HR=0.80; p=0.006) (Table 3).23 In the follow-up analyses, median OS was shown to be significantly longer with 500 mg of fulvestrant than with 250 mg (26.4 months versus 22.3 months; HR=0.81; p=0.016) (Table 3).24 Following the publication of these results, fulvestrant at 500 mg has become the standard dose for this drug.
A phase II, randomised, open-label study (Fulvestrant 500 mg Vs Anastrozole 1 mg in first line [FIRST] trial) compared fulvestrant 500 mg with anastrozole 1 mg as first-line endocrine therapy for postmenopausal women with hormone-receptor positive advanced breast cancer.25,26 A total of 205 patients were randomised to receive either standard fulvestrant 500 mg regimen or anastrozole 1 mg tablet.
The fulvestrant 500 mg arm demonstrated a statistically significant increase in the median time to progression and median overall survival (see Table 4). A consistent OS treatment effect was observed across predefined subgroups. This data supports superior efficacy of fulvestrant 500 mg over anastrozole as first-line endocrine therapy for postmenopausal women with hormone-receptor positive locally advanced or metastatic breast cancer. Although the results of the FIRST trial are encouraging, the definitive answer as to whether fulvestrant is the optimal first-line treatment will come from the ongoing placebocontrolled phase III FALCON trial that is randomising patients with oestrogen-receptor positive metastatic breast cancer to either 500 mg of fulvestrant or 1 mg of anastrzole.27
Arakai et al.28 reported a retrospective evaluation in 194 postmenopausal patients with recurrent/advanced breast cancer who received fulvestrant500 mg regimen from January 2012 to December 2014. The authors reported that OS was significantly longer in patients without prior chemotherapy than in patients with prior chemotherapy (p=0.0131). Time to treatment failure (TTF) was significantly longer in patients with less than two prior chemotherapy regimens (p=0.0093), de novo metastatic disease (p=0.0124) and without liver metastasis (p=0.0024).
Niraula et al.29 reported a meta-analysis of eight randomised controlled trials that evaluated fulvestrant compared to either tamoxifen or an AI. A total of 4,024 patients (2,032 on fulvestrant and 1,992 on control arms) with TTP/PFS was the primary endpoint. There was significant improvement in the TTP in the patients receiving fulvestrant who had visceral metastasis (HR=0.86; p<0.01), measurable disease (HR=0.74; p=0.01), and HER-2 overexpression (HR=0.43; p<0.001) in comparison with an AI or tamoxifen. Patients with hormone responsive advanced breast cancer that have visceral disease, measurable disease, or HER-2 driven disease would derive higher benefits from treatment with fulvestrant compared to tamoxifen or an AI.
A meta-analysis of clinical benefit rates (CBR) for fulvestrant 500 mg versus alternative therapies for treatment of postmenopausal, oestrogen-receptor positive advanced breast cancer evaluating five randomised controlled trials was presented by Robertson et al.30 The results indicate that fulvestrant 500 mg is associated with a significant improvement in CBR of approximately 34% compared with comparator endocrine therapies (OR=1.34; p=0.001).
Telford et al. conducted a network meta-analysis (NMA) of ten randomised controlled trials and compared OS and serious adverse events (SAEs) of fulvestrant 500 mg with alternative therapies for second-line treatment of postmenopausal, oestrogen-receptor positive advanced breast cancer.31 Fulvestrant 500 mg was associated with an improved OS versus fulvestrant 250 mg (HR=0.74), anastrozole (HR=0.73) and letrozole (HR=0.69), whereas the OS was similar with exemestane (HR=0.93) and everolimus plus exemestane (HR=1.02).
In a network meta-analysis comparing the efficacy of everolimus plus exemestane with that of fulvestrant 250 mg and 500 mg in the advanced breast cancer setting following adjuvant or first-line endocrine therapy, it was reported that that everolimus plus exemestane was more efficacious in terms of PFS/TTP than both fulvestrant 250 (HR=0.47) and 500 mg (HR=0.59). In the subgroup analysis of patients who received prior AI therapy everolimus plus exemestane was more efficacious for PFS/TTP than fulvestrant 250 mg and 500 mg (HR=0.47 and HR=0.55, respectively).32 However, treatment related serious adverse events were lower in the patients on fulvestrant 500 mg than on exemestane plus everolimus (2.2% versus 11%).24,33
Fulvestrant in combination with other hormonal therapies
Preclinical data suggests that fulvestrant may be more effective in a low-oestrogen environment.34 Bergh et al. conducted an open-label, randomised phase III trial – Fulvestrant and Anastrozole Combination Therapy (FACT) – in which a total of 514 postmenopausal women, or premenopausal women treated with gonadotropin-releasing hormone agonist, with advanced hormone receptor-positive breast cancer, were randomly assigned to receive fulvestrant (500 mg on day 1, and 250 mg on days 15, 29 and thereafter every four weeks) in combination with anastrozole (1 mg per day) or anastrozole alone at the same dosage.35
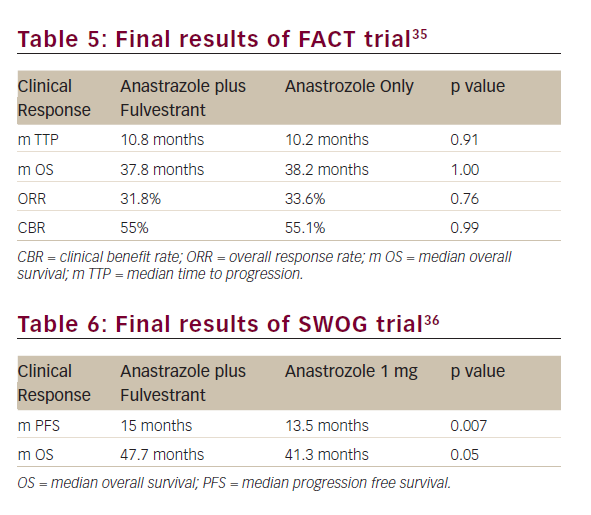
All the efficacy outcomes evaluated such as TTP, ORR, CBR, duration of response and OS were similar between both treatment arms (see Table 5).
The Southwest Oncology Group (SWOG) conducted a similar randomised phase III trial in which 707 postmenopausal women with hormone receptor-positive metastatic breast cancer, without prior chemotherapy or immunotherapy for metastatic disease, were randomised to fulvestrant plus anastrozole or anastrozole alone at the same doses as the FACT trial.36
The median progression-free survival (PFS) was 15.0 months and 13.5 months in the combination and the anastrozole arms (p=0.007), respectively. In patients who did not receive prior tamoxifen therapy the differences were even higher (17.0 months versus 12.6 months HR=0.7; p=0.006), whereas in 280 women (40.3%) previously treated with tamoxifen, it did not achieve statistical significance (13.5 months versus 14.1 months, respectively; HR=0.89; p=0.37). OS was also longer in patients treated with the combination than in those who received anastrozole alone (47.7 months versus 41.3 months, respectively; HR=0.81; p=0.05), despite the fact that 41% of patients in the anastrozole arm crossed over to fulvestrant after progression (Table 6).
The differences between trials could explain the discrepancy in results. In the SWOG trial 40% of patients had prior exposure to adjuvant tamoxifen, whereas this percentage rose to 70% in the FACT trial. Moreover, in the SWOG trial when patients were stratified according to prior tamoxifen exposure, only patients without previous tamoxifen showed statistically significant differences in terms of PFS.
Johnston et al.37 conducted a multicentre, phase III randomised controlled trial in postmenopausal women with hormone-receptor positive breast cancer with relapse or progression on a non-steroidal aromatase inhibitor (NSAI) (given as adjuvant for at least 12 months or as first-line treatment for at least six months). Participants were randomly assigned (1:1:1) to receive fulvestrant (500 mg intramuscular injection on day 1, followed by 250 mg doses on days 15 and 29, and then every 28 days) plus daily oral anastrozole (1 mg); fulvestrant plus anastrozole-matched placebo; or daily oral exemestane (25 mg). A total of 723 patients underwent randomisation: 243 were assigned to receive fulvestrant plus anastrozole, 231 to fulvestrant plus placebo. Median PFS was 4.4, 4.8 and 3.4 months in patients assigned to fulvestrant plus anastrozole, fulvestrant plus placebo, and exemestane, respectively. There was no significant difference between the patients assigned to fulvestrant plus anastrozole and fulvestrant plus placebo (p=0.98), or between those assigned to fulvestrant plus placebo and exemestane (p=0.56). The authors concluded that after loss of response to NSAIs in postmenopausal women with hormone-receptor positive advanced breast cancer, treatment with fulvestrant combined with oestrogen deprivation is no better than either fulvestrant alone or exemestane.
Cristofanilli et al.38 reported final analysis of the a phase III study, PALOMA-3, involving 521 patients with advanced hormone-receptor positive breast cancer that had relapsed or progressed during prior endocrine therapy. Patients were randomised in a 2:1 ratio to receive palbociclib and fulvestrant or placebo and fulvestrant. Overall, median PFS was longer for patients receiving the combination of palbociclib and fulvestrant versus fulvestrant alone (9.5 months versus 4.6 months; HR=0.46, p<0.0001); the patients receiving the combination had a 54% lower risk for disease progression. Most of the adverse events of grade 3 or 4 that occurred with the combination were neutropenia (65% versus 1% for fulvestrant); febrile neutropenia was uncommon. Leukopenia also occurred at a greater frequency in patients receiving the combination (28% versus 2% for fulvestrant). On the basis of data from the PALOMA-3 study, the US Food and Drug Administration (FDA) expanded the use of palbociclib in combination with fulvestrant for the second-line treatment of advanced or metastatic breast cancer.39
The recently presented FERGI trial40 was the first randomised phase II study to evaluate the combination of ET with a PI3KCA inhibitor in postmenopausal women with AI resistant HR-positive advanced breast cancer. The results showed that adding the PI3K inhibitor pictilisib to fulvestrant in women with postmenopausal oestrogen receptor (ER)- positive, HER2-negative breast cancer improved progression-free survival (PFS) by a median of 1.5 months. The PFS was 6.6 months in the combination arm compared with 5.1 months in the fulvestrantalone arm (HR=0.74; p=0.0959). In an exploratory subgroup analysis, the patients that benefited most from the combination therapy had both ER-positive and progesterone receptor (PR)-positive disease. The PFS was 7.4 months and 3.7 months in the combination and placebo arms, respectively (p=0.002).
Lipid-lowering action of fulvestrant
Camerini et al. reported that total cholesterol levels significantly decreased during treatment with fulvestrant (219.8 +/- 45.3 versus 201.4 +/- 42.1 mg/dl; p = 0.0054) together with LDL-cholesterol (129.7 +/- 41.39 versus 112.3 +/- 37.1 mg/dl; p=0.018) in a study conducted on 51 patients with HR-positive metastatic breast cancer. The lipid lowering effect of fulvestrant possibly related to an influence on lipid metabolism by a mechanism in which a role could be played by progesterone receptor.41
Safety of fulvestrant
In the European and American trials (Trial 0020 and Trial 0021) that compared fulvestrant 250 mg with anastrozole, both treatments were well tolerated.42–44 Anastrazole-receiving patients had a higher incidence of joint disorders, including arthralgia, arthrosis and arthritis (p=0.0234). Other adverse events like nausea (26.0% versus 25.3%), asthenia (22.7% versus 27.0%), pain (18.9% versus 20.3%), vasodilation (17.7% versus 17.3%), and headache (15.4% versus 16.8%) were similar across the fulvestrant group and the anastrozole group, respectively. Withdrawals due to drug- related adverse events were 0.9% and 1.2% in the fulvestrant arm and the anastrozole arm, respectively.
The CONFIRM trial showed that both doses of fulvestrant (250 mg and 500 mg) were well tolerated with no substantial differences in the incidence and severity of prespecified adverse events. There were no significant differences in the quality of life between both dosages.23
The FIRST trial showed that fulvestrant 500 mg was well tolerated with an adverse events profile comparable to that of anastrozole and consistent with that previous evidence.12,23,25 The incidence of serious adverse events was 11.9% versus 9.7% with fulvestrant 500 mg and anastrozole, respectively. The most common adverse events in the fulvestrant 500 mg arm were bone pain (13.9%), nausea (10.9%), arthralgia (9.9%), constipation (9.9%), vomiting (8.9%) and dyspnoea (8.9%). The incidence of arthralgia was similar between the two arms, but headache and asthenia were less frequent in the fulvestrant arm.
In a meta-analysis by Telford et al., fulvestrant 500 mg exhibited decreased SAE rates compared to letrozole, exemestane, and everolimus plus exemestane (13.22 versus 20.65, 46.63, 67.30 events, respectively) in base case and versus fulvestrant 250 mg and letrozole (14.88 versus 15.67, 23.41 events, respectively) in the anti-oestrogen subgroup.31
Other trials, such as the EFECT and the FACT trials, showed no differences in terms of safety data of fulvestrant in comparison with the control drugs.10,26 Only the FACT trial showed a higher incidence of hot flashes in the combination arm compared with the single treatment arm (p=0.003).
In general, fulvestrant is well tolerated, with very low frequency of treatment dropout. In addition, there were no significant differences between the toxicity profiles of fulvestrant and other hormonal therapies such as anastrozole, tamoxifen and exemestane, or between both doses of fulvestrant in the treatment of hormone-sensitive advanced breast cancer.
Current role of fulvestrant in therapy and guideline recommendations
The clinical efficacy of fulvestrant in postmenopausal women with hormone receptor-positive metastatic breast cancer who had progressed on prior anti-oestrogen therapy was established for a 250 mg once-monthly regimen by a retrospective analysis of combined data from two large randomised trials (0020 and 0021).
Although the fulvestrant 250 mg monthly regimen is clearly effective, steady-state concentrations of the drug are only reached following three to six months of treatment,45 which potentially allows the possibility of an early relapse. In contrast, steady-state concentrations for the fulvestrant 500 mg regimen are reached within one month and systemic exposure is approximately dose proportional compared with the 250 mg dose. Results from the phase III CONFIRM trial clearly demonstrated a clinically relevant increased efficacy of the fulvestrant 500 mg regimen compared with the 250 mg monthly regimen.24 This led to the approval of fulvestrant 500 mg regimen as a standard secondline therapy in HR-positive metastatic breast cancer in postmenopausal women with disease progression following anti-oestrogen therapy.
Fulvestrant was also shown to be effective in breast cancer patients previously untreated with hormonal therapy (FIRST trial) with a higher clinical benefit in the form of greater increase in OS and PFS than anastrozole. Hence, the National Comprehensive Cancer Network (NCCN)46 and the European School of Oncology (ESO) and European Society of Medical Oncology (ESMO) guidelines47 recommend use of fulvestrant both in first-line (i.e. treatment naive) and second-line (i.e. prior endocrine therapy) treatment for patients with advanced breast cancer. The NCCN also has provided a category 1 recommendation for the use of the combination of palbociclib and fulvestrant in postmenopausal women or for premenopausal women receiving luteinizing-hormonereleasing hormone agonist. The FDA recently approved the use of palbociclib in combination with fulvestrant for the second-line treatment of advanced or metastatic breast cancer. The FALCON study, a multicentre, double-blind, randomised, phase III trial is currently ongoing to compare the efficacy in terms of PFS and tolerability of fulvestrant 500 mg with anastrozole 1 mg as endocrine treatment for postmenopausal women with hormone-receptor positive locally advanced or metastatic breast cancer who have not previously been treated with any hormonal therapy (Clinicaltrials.com identifier NCT01602380).48 This trial could lead to the FDA approval of fulvestrant 500 mg as first line endocrine therapy if results correlate with the phase II FIRST trial.
Endocrine therapy remains the mainstay of first-line treatment for postmenopausal women with HR-positive HER2-negative advanced breast cancer. Fulvestrant as monotherapy and also in combination with newer agents has established efficacy and good tolerability in patients of advanced or metastatic breast cancer whose disease progress after prior endocrine therapy. Based on data from FIRST and FALCON trial, fulvestrant may also become an option as first-line endocrine therapy. Endocrine agents in monotherapy demonstrated high efficacy and tolerability, but endocrine resistance commonly arises. Currently, there is an urgent need to develop predictive tools or markers that can reliably identify patients who will benefit from endocrine therapy alone and those that will require different approaches, such as poly-endocrine therapy or combination with targeted agents. Studies investigating poly-endocrine therapy are contradictory and need further validation. Numerous new agents in combination with endocrine therapy are in clinical development for patients with HR-positive advanced breast cancer. However, while considering such combination therapy, any additional benefit should be carefully weighed against additional toxicity and costs













