Since the approval of palbociclib in 2015,1 cyclin dependent kinase (CDK) 4/6 inhibitors have been a heavily-researched and discussed class of medication for the management of metastatic, hormone receptor positive (HR+), human epidermal growth factor receptor 2-unamplified (HER2-) breast cancer. Because their approval and rise to prominence was based on benefit seen in progression-free survival (PFS), the ideal sequencing of therapy has been debated amongst oncologists. More recently, overall survival (OS) data from several studies have matured, hopefully putting to rest concerns about the appropriateness of CDK 4/6 inhibition in the first-line setting. This article will review the evolution of systemic therapy for advanced HR+ breast cancer leading up to the development and rise of CDK 4/6 inhibitors, followed by an overview and discussion of the recently reported survival data in these studies.
Evolution of systemic therapy for metastatic, hormone receptor positive breast cancer
In the textbook “Popular Medicine or The American Family Physician” published by Drs. George Capron and David Slack in 1854, the entry on “Cancer” comprises 2.5 pages in a 705-page tome, and breast cancer is the paradigm.2
“The scirrhous… is a hard and irregular lump, which forms under the skin, in the breasts of women” and when “attended with a burning, shooting pain and the skin over it has become dusky, purple, or livid, it has become a confirmed cancer. […] The axillary glands and the arms will swell and become stiff and hard, and the whole body will partake of the disease. […] In some, the progress of the disease is rapid, and in others, exceedingly slow. […] Almost the only hope of a cure in this disease is found in having it removed by the knife. […]”
Fortunately, astute observers of the time noted that the size of some breast tumors changed during the menstrual cycle and that progression of disease might slow if a woman with breast cancer underwent menopause, leading to the hypothesis that ovarian hormones were linked to breast cancer progression. Dr George Thomas Beatson of Glasgow Cancer Hospital published the results of bilateral oophorectomy in three women in The Lancet in 1896.3 Having miraculously survived surgery in the late 19th century, one of his patients, a pre-menopausal, 40-year-old woman with inoperable breast cancer, had complete remission of disease and lived for another 4 years.
Professor Elwood Jensen’s work, defining the role of estrogen and estrogen receptors in the 1950s–1970s, set the stage for development of targeted treatment for estrogen receptor positive breast cancer,4,5 the first of which was tamoxifen (Figure 1). In the 1980s, the use of tamoxifen prior to chemotherapy, with chemotherapy, or after chemotherapy in women with advanced breast cancer was explored in clinical trials with results showing that although the overall response rate to first-line cytotoxic chemotherapy is higher than that of tamoxifen, OS is virtually the same if chemotherapy is given prior to tamoxifen, tamoxifen prior to chemotherapy, or if tamoxifen and chemotherapy are given in combination.6–9 Being far less toxic compared to cytotoxic chemotherapies (prior to the advent of powerful anti-emetics and granulocyte growth factors), tamoxifen became a preferred first-line therapy.
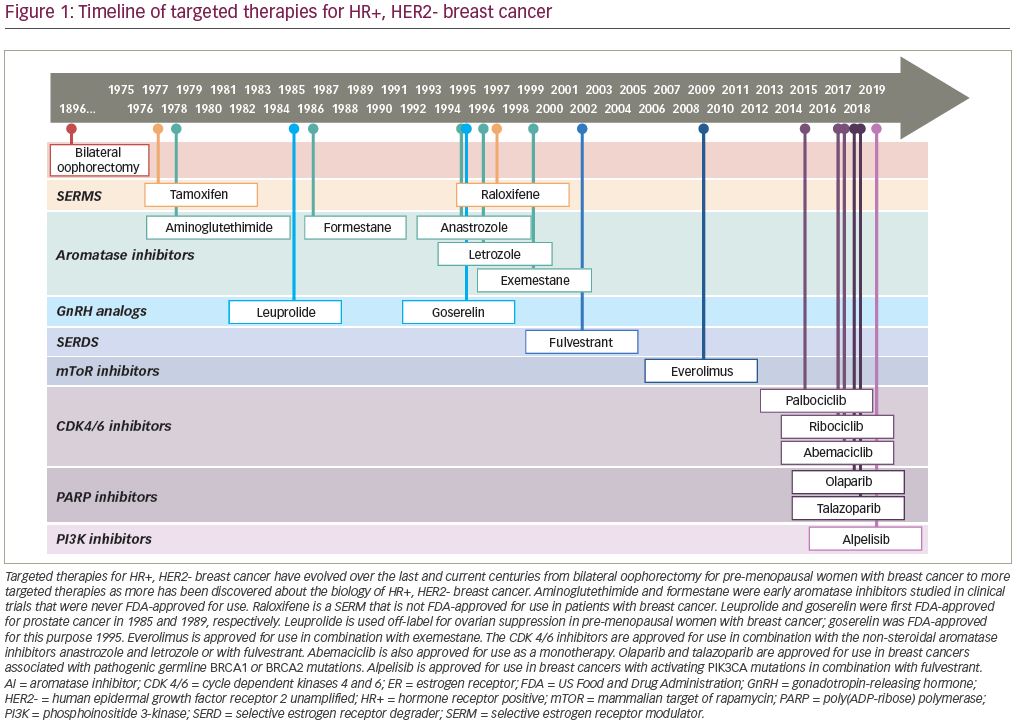
Aromatase inhibitors were originally studied and developed for clinical use by Harry and Angela Brodie, who were studying the enzyme, aromatase.10 Aromatase inhibitors block estrogen biosynthesis by preventing the peripheral conversion of androgens to estrogens by the enzyme aromatase. The current generation of aromatase inhibitors are anastrozole, letrozole, and exemestane, with anastrozole being US Food and Drug Administration (FDA)-approved in 1995, letrozole in 1996, and exemestane in 1999. These aromatase inhibitors improved upon the tolerability and ease of administration of earlier generations of aromatase inhibitors.11,12 The use of aromatase inhibitors in pre-menopausal women requires chemical ovarian suppression, oophorectomy, or ablation by radiation. Goserelin, a gonadotropin-releasing hormone analog, causes estrogen depletion via suppression of the hypothalamic–pituitary–gonadal hormone axis, and was approved for use in pre-menopausal women with breast cancer in 1995.13–15 All three aromatase inhibitors have been evaluated in phase III clinical trials in post-menopausal patients with HR+, HER2- breast cancer in the first-line metastatic setting versus tamoxifen, with results demonstrating superiority in terms of median PFS; however, there are no clinical trials demonstrating the superiority of one aromatase inhibitor over another.16–21
Fulvestrant is a selective estrogen receptor degrader/downregulator administered by depot intramuscular (IM) injection with a first-month loading dose followed by a monthly maintenance dose. FDA-approved in 2002 for use in the HR+, HER2- metastatic setting, fulvestrant was originally studied in clinical trials at a maintenance dosage of 250 mg IM monthly, which in 2010 was found to be suboptimal to a maintenance dosage of 500 mg IM monthly.22 In the interim period between 2002–2010, fulvestrant 250 mg IM monthly was found to have efficacy similar to tamoxifen 20 mg by mouth daily in the first-line setting,23 anastrozole 1 mg by mouth daily in the second-line setting,24,25 and exemestane 25 mg by mouth daily in the second-line setting.26 The FALCON study, published in 2016, compared fulvestrant 500 mg IM every 2 weeks x2 doses followed by 500 mg every 28 days to anastrozole 1 mg by mouth daily in the first-line metastatic HR+, HER2- setting, demonstrating a statistically significant median PFS of 16.6 months in the fulvestrant group versus 13.8 months in the anastrozole group.27
In 2012, the concept of combining endocrine therapy with an additional molecular target was ushered in by the BOLERO-2 trial. BOLERO-2 was a phase III clinical trial studying the combination of everolimus, a mammalian target of rapamycin (mTOR) inhibitor, with exemestane in the second-line HR+, HER2- metastatic setting after progression on a non-steroidal aromatase inhibitor (NSAI). The median PFS was 6.9 months with everolimus + exemestane versus 2.8 months for placebo + exemestane (p<0.001).28 However, poor tolerability of everolimus stunted widespread clinical adoption. Subsequent molecularly targeted agents, such as poly(ADP-ribose) polymerase (PARP) inhibitors olaparib and talazoparib as well as the PI3 Kinase inhibitor alpelisib, have been shown to be effective in specific germline (BRCA 1/2) or somatic (PIK3CA) mutations, respectively.29,30 While these agents are not options for all patients with HR+, HER2- breast cancer, they are good options for those who are candidates.
Outcome measures for first-line chemotherapy in metastatic HR+, HER2- breast cancer are not as clear as those for hormonal therapies because (1) triple negative and HR+, HER2- breast cancers have typically been combined into one group of “HER2-negative” breast cancers, and (2) many or most of the HR+, HER2- patients have previously been exposed to hormonal therapies in the metastatic setting (i.e., the cytotoxic chemotherapy is not truly first-line). For example, the RIBBON-1 trial directly compared common and currently-used cytotoxic therapies—capecitabine, taxane (docetaxel or nab-paclitaxel), or anthracycline-based therapy (epirubicin or doxorubicin with cyclophosphamide plus or minus fluorouracil)—with or without the addition of bevacizumab in the “first-line” metastatic setting in persons with HER2- breast cancer.31 The taxane and anthracycline cohorts were pooled. Approximately 75% of the patients were HR+ in both cohorts, with over half of the capecitabine patients and more than one-third of the taxane/anthracycline cohort already having received hormonal therapy in the metastatic setting. Median PFS was 5.7 months with capecitabine (n=206) and 8.0 months in the combined taxane or anthracycline-based chemotherapy arm (n=207). Patients in the anthracycline cohort had not had previous exposure to anthracycline.
Rise of CDK 4/6 inhibitors
Though endocrine therapy had been the mainstay of treatment for HR+ breast cancer for decades, eventual endocrine resistance was almost universal. Alterations of key checkpoints in the cell cycle, including the CDK 4/6 pathway, were identified to be commonly associated with resistance to endocrine therapy.32 Within the cell cycle, the transition between G1 and S phase is heavily regulated, and only occurs if the cell can proceed past the restriction point, or the “R point”.33 In its active, de-phosphorylated state, retinoblastoma protein (Rb) prevents this transition. CDK 4 and CDK 6, when associated with cyclin-D, regulate Rb by hyperphosphorylating and de-activating it, thereby allowing progression through the cell cycle. CDK 4/6 inhibitors restrict progression through the cell cycle by preventing hyperphosphorylation of Rb, resulting in an active form of Rb that can effectively restrict progression through the cell cycle.34
Pre-clinical studies by Finn et al. suggested that HR+ breast cancer cell lines were sensitive to growth inhibition with a CDK 4/6 specific inhibitor, palbociclib, in both endocrine-sensitive and endocrine-resistant cell lines.35 This growth inhibition was synergistic with endocrine therapy. While the majority of palbociclib-sensitive cell lines were HR+, some HER2+ and triple-negative cell lines exhibited varying degrees of sensitivity to palbociclib.
These promising pre-clinical findings led to the development of randomized, controlled clinical trials with CDK 4/6 inhibitors in the first- and second-line metastatic HR+ HER2- breast cancer settings. First-line studies, such as PALOMA-236 (palbociclib), MONALEESA-237 (ribociclib), and MONARCH-338 (abemaciclib), randomized post-menopausal women who had not received prior endocrine therapy in the metastatic setting to receive either a NSAI plus either a CDK 4/6 inhibitor or placebo. Importantly, 22% of patients in PALOMA-2 had a disease-free interval of less than 12 months, as compared to 2% of patients in MONALEESA-2 and 0% in MONARCH-3. MONALEESA-7 was the only first-line study to compare endocrine therapy with endocrine therapy + a CDK 4/6 inhibitor (ribociclib) in pre-menopausal patients.39 The second-line studies, such as PALOMA-3,40 MONALEESA-3,41 and MONARCH-2,42 all randomized patients who had progressed or relapsed on endocrine therapy to receive fulvestrant plus either placebo or a CDK 4/6 inhibitor. MONALEESA-3 only allowed post-menopausal women and men, while PALOMA-3 and MONARCH-2 also included pre-menopausal women. Additionally, MONALEESA-3 also included patients who were treatment naïve in the metastatic setting. A fourth trial in the second-line setting, MONARCH-1,43 was the only larger trial to investigate a CDK 4/6 inhibitor as a single agent after progression on prior chemotherapy and endocrine therapy.
PALOMA-2 and PALOMA-3 both showed improved PFS when palbociclib was added to first-line letrozole (median PFS 24.8 versus 14.5 months, hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.46–0.72, p<0.001) and second-line fulvestrant (PFS 9.2 versus 3.8 months, HR 0.42, 95% CI 0.32–0.56, p<0.001), respectively.36,40 MONALEESA-2 and MONALEESA-3 similarly showed improved PFS with the addition of ribociclib to first-line letrozole (18 month PFS rate 63% versus 42.2%, HR 0.56, 95% CI 0.43–0.72, p<0.001) and first- or second-line fulvestrant (median PFS 20.5 versus 12.8 months, HR 0.59, 95% CI 0.48–0.73, p<0.001), respectively.37,41 MONARCH-1 evaluated single-agent abemaciclib in a single-arm, phase II trial of abemaciclib in heavily pre-treated patients with advanced HR+ breast cancer, showing a meaningful clinical benefit rate (42.4%) and objective response rate (19.7%).43 Based on MONARCH-1, abemaciclib is the only CDK 4/6 inhibitor approved as monotherapy.44 MONARCH-2 and MONARCH-3 were randomized phase III trials showing improved PFS when abemaciclib was added to second-line fulvestrant (median PFS 16.4 versus 9.3 months, HR 0.55, 95% CI 0.45–0.68, p<0.001) and first-line NSAI (median PFS not yet reached versus 14.7 months, HR 0.54, 95% CI 0.41–0.82, p<0.001), respectively.38,42 Across these studies, all subgroups seemed to derive benefit. Interestingly, patients with traditionally high-risk features such as high grade, visceral involvement, and younger age, seemed to show benefit of CDK 4/6 inhibition most consistently. MONALEESA-7 was unique in that its patient population was exclusively pre-menopausal, and also showed significant PFS benefit when ribociclib was added to either tamoxifen or a NSAI + ovarian suppression (median PFS 23.8 versus 13.0 months, HR 0.55, 95% CI 0.44–0.69, p<0.001).39
Overall survival with CDK 4/6 inhibitors
These studies led to the widespread approval of palbociclib, ribociclib, and abemaciclib by regulatory agencies and the subsequent widespread adoption of these agents in the first-line metastatic setting. Over the last 2 years, OS data from some of these trials have matured (summarized in Table 1). The first trial to report on OS data was PALOMA-3.45 The prespecified threshold of significance, taking into account a planned interim analysis, was a two-sided p-value of 0.047. The median OS in the palbociclib–fulvestrant group was longer than that of the placebo–fulvestrant group (34.9 versus 28.0 months). This 6.9-month absolute difference translated into a HR for death of 0.81 with a 95% CI 0.64–1.03 and a p-value of 0.09, and was therefore not considered statistically significant. The median time to receipt of chemotherapy, however, was significantly improved in the fulvestrant–palbociclib group (17.6 versus 8.8 months, HR 0.58, 95% CI 0.47–0.73, p<0.001).
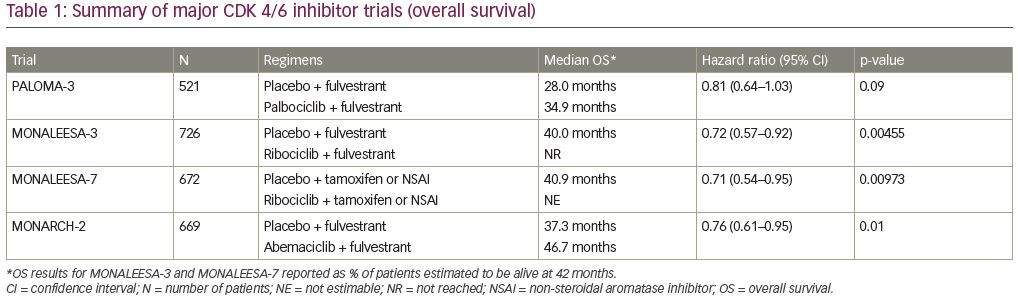
Subsequently, two trials with ribociclib reported significantly improved OS. First, MONALEESA-7 showed a 24.2% absolute increase in 42-month OS rate, with median OS not yet being reached in the ribociclib group at a median duration of follow-up of 34.6 months.46 This survival benefit held true across all subgroups. Interestingly, 18.6% of patients in the placebo group subsequently received a CDK 4/6 inhibitor, with time to progression on second-line therapy also being significantly longer in the ribociclib group.
Shortly after these results were presented at the 2019 American Society of Clinical Oncology annual meeting, the OS results of MONALEESA-3 were announced at the 2019 annual European Society of Medical Oncology (ESMO) meeting. Similarly, the ribociclib group in MONALEESA-3 experienced significantly improved 42-month survival rate (57.8% versus 45.9%, HR 0.72, 95% CI 0.57–0.92, p=0.00455).47 MONALEESA-3 is unique among these studies in that it allowed patients in both the first- and second-line setting, giving insight into how two different populations responded to an intervention, which would typically require a cross-trial comparison. A subgroup analysis of the first-line population showed a 10.1% increased OS benefit, with an updated median PFS of 33.6 months in the ribociclib group versus 19.2 months in the fulvestrant-alone group (HR 0.55, 95% CI 0.42–0.72).
Concurrent with the results of MONALEESA-3, MONARCH-2 also reported OS data at ESMO 2019. The addition of abemaciclib to fulvestrant in the second-line setting was associated with significantly improved median OS (46.7 versus 37.3 months, HR 0.757, 95% CI 0.606–0.945, p=0.01).48 Subgroups with especially improved survival were those with visceral disease as well as those with primary resistance to endocrine therapy, and all subjects in the abemaciclib arm also experienced improved time to chemotherapy, time to second progression, and chemotherapy-free survival. A meta-analysis of data from PALOMA-3, MONALEESA-3, and MONARCH-2 confirmed that OS, as well as PFS, is superior in patients receiving a CDK 4/6 inhibitors + fulvestrant as compared to fulvestrant alone (HR 0.77, 95% CI 0.67–0.89, p<0.001).49 A second meta-analysis of the PALOMA 1, 2, and 3; MONALEESA 2, 3, and 7; and MONARCH 2, 3, and MONARCH plus, additionally showed that the addition of a CDK 4/6 inhibitor to endocrine therapy improved overall survival, regardless of menopausal status, endocrine resistance, visceral versus bone only disease, and age.50
Since the approval of CDK 4/6 inhibitors, oncologists have questioned the appropriate sequencing of endocrine therapy in the metastatic setting. Is it best to start with aromatase inhibitor monotherapy and then add CDK 4/6 inhibition upon progression, or to start with the combination at the outset? Prior to the CDK era, everolimus was the only approved agent that could be added to endocrine therapy, and it was common to utilize this only upon progression after NSAI and fulvestrant. The major reason for this choice of sequencing was due to the substantial side effect profile of everolimus. Additionally, BOLERO-2 did not show significant OS benefit with the addition of everolimus.51 Given the lack of OS benefit and more challenging side effect profile, adding mTOR inhibition seemed appropriate to delay until a later line of therapy.
However, with multiple studies now showing OS benefit of CDK 4/6 inhibition, especially with two studies showing benefit in the first-line setting, all patients should receive these drugs in combination with endocrine therapy for first-line treatment of metastatic, HR+, HER2- breast cancer. Additionally, the comparatively favorable and manageable side effect profile of these drugs facilitates first-line treatment, even in patients who are asymptomatic from their disease.52 With palbociclib and ribociclib, the most common adverse event is neutropenia, which is largely asymptomatic, and very rarely associated with neutropenic fever.52 Dose modification for neutropenia does not seem to impact survival outcomes,53 and palbociclib-associated neutropenia may in fact be associated improved PFS (though more data are needed to confirm this).54
While it is clear that these agents should be used in the first-line metastatic setting, several important clinical questions remain unanswered. Firstly, since the OS benefit seen in PALOMA-3 was not statistically significant, can the OS benefit seen with ribociclib and abemaciclib be extrapolated to palbociclib? While the Kaplan–Meier PFS curves were nearly interchangeable, suggesting equivalent efficacy, the three drugs differ in their chemical structure and have different side effect profiles (i.e. QT prolongation with ribociclib, more diarrhea with abemaciclib). Therefore, it is possible that the observed differences in OS are related to these varying biochemical properties. However, it is important to keep in mind that while the OS benefit seen in PALOMA-3 was not statistically significant, it bordered on significance, and differences in outcomes could also be explained by differing patient populations in the two trials. Additionally, a real-world retrospective comparative effectiveness analysis of the Flatiron Health database by DeMichele and colleagues did show a statistically significant difference in OS in patients receiving first-line palbociclib and letrozole (n=772, 2 year OS rate 80.1%) compared with first-line letrozole alone (n=658, 2 year OS rate 63.9%).55
A second unanswered question is what to do about patients presenting with significant visceral disease burden. Traditionally, cytotoxic chemotherapy has been considered the standard of care for these high-risk patients in need of a rapid response, and remains the recommended treatment modality for patients in visceral crisis.56 However, it is not clear that chemotherapy is superior to hormone therapy + a CDK 4/6 inhibitor in patients with visceral disease without visceral crisis. Giuliano et al. recently published a systemic review and meta-analysis for endocrine treatment versus chemotherapy in post-menopausal women with HR+, HER2- metastatic breast cancer in the first- or second-line.57 They concluded that there is no chemotherapy regimen that is significantly better than hormone therapies in combination with CDK 4/6 inhibitors in terms of median PFS. Additionally, the only regimen with a significantly better overall response than aromatase inhibitor + CDK 4/6 inhibitor was paclitaxel + bevacizumab, a regimen no longer in clinical use. While the Schettini meta-analysis also suggested that CDK 4/6 + endocrine therapy showed improved OS benefit compared with endocrine therapy alone in visceral disease, the studies in this analysis did not include chemotherapy.50 The Young-PEARL study was a small phase II trial randomizing pre-menopausal patients to either capecitabine or palbociclib, exemestane, and leuprolide.58 There was no significant difference in overall response rate between the two arms, and in patients with visceral disease there was no difference in PFS. In patients without visceral disease, median PFS was significantly improved in the palbociclib arm compared to the capecitabine arm (20.7 versus 14.4 months, HR 0.496, 95% CI 0.26–0.94, p=0.0272). The larger PEARL trial similarly compared endocrine therapy + palbociclib versus capecitabine, and found no difference in PFS or OS between the two arms.59 An ongoing trial (ClinicalTrials.gov identifier: NCT04031885) is studying abemaciclib + fulvestrant versus chemotherapy of physician’s choice exclusively in patients with visceral metastases.
Much lies ahead on the horizon for CDK 4/6 inhibitors. Currently, data are insufficient to recommend continued CDK 4/6 inhibition beyond progression on a CDK 4/6 inhibitor. The TRINITI-1 trial did show a promising clinical benefit rate of 40% in patients receiving ribociclib, everolimus, and exemestane who had progressed on prior CDK 4/6 inhibitor.60 The ongoing PACE trial (ClinicalTrials.gov identifier: NCT03147287) will hopefully shed further light on the role of continuing CDK 4/6 inhibitors beyond progression. Another important, and as of yet unanswered, question is whether or not any biomarkers exist that predict response or resistance to CDK 4/6 inhibitors. Aside from hormone receptor positivity and HER2 negativity, there does not seem to be any clear biomarkers that predict response to these drugs. Biomarkers in the cell-cycle pathway have been suggested as natural candidates to predict response.61 A small, retrospective study of two single-arm phase II trials of single-agent palbociclib suggested that early grade 1+ neutropenia associated with single-agent palbociclib was associated with improved PFS in both breast and non-breast cancers.55 However, early neutropenia does not seem to predict response to abemaciclib,62 and data from PALOMA-3 did not suggest a PFS difference with development of grade 3+ neutropenia.54
Finally, much work has been done, and is still ongoing, with CDK 4/6 inhibitors in early stage breast cancer. The large, international, phase III PALLAS, monarchE, and NATALEE trials will explore whether or not the addition of palbociclib, abemaciclib, and ribociclib (respectively) to adjuvant aromatase inhibitor-based endocrine therapy will improve invasive disease-free survival.63–66 These trials differ slightly with regard to eligibility criteria, duration of CDK 4/6 inhibitor administration, and starting dose of CDK 4/6 inhibitor, and have the potential to change the face of adjuvant endocrine therapy for early-stage HR+ breast cancer. Recently, press releases from PALLAS and monarchE suggest conflicting results with regard to the benefit of adding CDK 4/6 inhibitors to adjuvant endocrine therapy. PALLAS has reported at the first pre-planned efficacy and futility analysis that the trial is unlikely to show any benefit of adding palbociclib to endocrine therapy.67 However, the monarchE trial is reporting that the addition of abemaciclib seems to result in improved invasive disease-free survival compared to endocrine therapy alone.68 The NATALEE trial is currently still enrolling and the initial results have yet to be reported.
The role, if any, of CDK 4/6 inhibitors in the neoadjuvant setting has yet to be defined. NeoPalAna and PALLET demonstrated that adding CDK 4/6 inhibition increases the rate of complete cell cycle arrest (Ki67 ≤2.7%), though no pathologic complete responses were seen in NeoPalAna, and no difference in the low pathologic complete response rate was seen between the letrozole versus palbociclib groups in PALLET.69,70 The neoMONARCH trial similarly combined a CDK 4/6 inhibitor (abemaciclib) with pre-operative endocrine therapy, and also found a greater change in Ki67 in the abemaciclib arm after 2 weeks of treatment, with a pathologic complete response rate of 4%.71 Interestingly, additional findings of neoMONARCH also demonstrated increased immune response activation, as well as downregulation of genes associated with estrogen response and cell-cycle pathways. The neoadjuvant CORALEEN study compared 24 weeks of neoadjuvant ribociclib/letrozole to neoadjuvant chemotherapy (dose dense doxorubicin and cyclophosphamide followed by weekly paclitaxel), and found comparable rates of “molecular downstaging” between the two arms, as defined by a decrease in the PAM50 risk of relapse from high to low risk.72 While these neoadjuvant studies have demonstrated changes at the molecular level, the clinical utility of neoadjuvant CDK 4/6 inhibitors is unclear, especially with most systemic therapy in early-stage HR+, HER2- breast cancer taking place in the adjuvant setting.
Conclusions
Systemic therapy for HR+, HER2- breast cancer has steadily evolved over the past several decades. While the relatively recent introduction of CDK 4/6 inhibitors has resulted in rapid adoption of these agents, many oncologists rightfully questioned whether or not it was appropriate to use them in the first-line setting or upon progression. OS reporting of MONALEESA-7, MONALEESA-3, and MONARCH-2 all show significantly improved survival when ribociclib or abemaciclib is added to endocrine therapy. While the results of PALOMA-3 suggested a non-significant trend towards benefit with the combination, this may be due to differences in patient population, and a recent real-world database analysis suggests that there may be an OS benefit with palbociclib. Most importantly, the findings from MONALEESA-7 and MONALEESA-3 included data for patients receiving the combination in the first line, suggesting that waiting to administer these medications until after progression may negatively impact OS. While the purpose of this article was to provide a historical overview of the important trials leading to the current standard of care for HR+, HER2- metastatic breast cancer, it is not a systematic review, though these findings have been confirmed in a prior systematic review and meta-analysis.50 These medications have helped patients live longer, with an acceptable side effect profile, and have changed the game in the treatment of metastatic breast cancer. Ongoing trials will hopefully identify biomarkers that may predict response to these drugs, as well as understand whether or not these agents will improve disease-free survival in early stage breast cancer, potentially helping more patients live free of this disease.













