The treatment of patients with chronic phase chronic myeloid leukaemia (CP-CML) has changed dramatically with the advent of the first BCR-ABL tyrosine kinase inhibitor (TKI) imatinib (Gleevec®, Novartis Pharmaceutical Corporation, New Jersey, US) in the 1990s.1,2 Multiple studies demonstrated the efficacy and acceptable tolerability of imatinib 400 mg daily.3,4 Despite the good cytogenetic and molecular response rates, some patients show primary resistance (refractoriness) or relapse after an initial response (secondary or acquired resistance).5,6 Adverse events occurred mainly within the first 2 years of treatment initiation and symptoms appeared to be mild or moderate in most instances, but with other treatment options available even low-grade toxicities are less acceptable and lead to discontinuation. Definitions of haematological, cytogenetic and molecular response have been previously described.7 Resistance in this setting has been defined as treatment with imatinib ≥600 mg/d (for ≥3 months) with disease progression (≥50% increase in white blood cells), or no haematological response after 4 weeks, or patients receiving <600 mg/d with mutations at any of the following ABL amino acids: L248, G250, Q252, Y253, E255, T315, F317, H396.7 Dasatinib (Sprycel®; Bristol-Myers Squibb, New Jersey, US) and nilotinib (Tasigna®; Novartis, New Jersey, US), two second-generation TKIs initially launched for use as second-line therapies in 2006/2007, were approved for first line use in 2010. Treatment with dasatinib or nilotinib in recommended doses of 100 mg daily or 400 mg twice daily resulted in significantly higher cytogenetic and molecular response rates compared to imatinib 400 mg daily but there was no progression-free survival (PFS) or overall survival (OS) benefit. With the success of TKIs the earlier gold standard of allogeneic haematopoietic stem-cell transplantation (allo-HSCT) is reserved for later-line patients failing to achieve adequate responses.
Second-line treatment
In second-line, dasatinib and nilotinib have been recommended for treatment of CP-CML patients with resistance/intolerance to imatinib. In addition, bosutinib (Bosulif®; Pfizer, New York, US) received a conditional marketing authorisation in 2013 that is valid throughout the European Union (see Table 1),8–10 for the treatment of adult chronic myeloid leukaemia (CML) patients in all phases.11,12 Despite some good responses to second-line TKI therapy, approximately half of patients treated with dasatinib 100 mg daily or nilotinib 400 mg twice daily develop resistance or intolerance8,13,14 and discontinue therapy.
Third-line treatment Patients who fail to respond to second-line therapy generally receive third-line therapy with another TKI,10,15–20 and identifying patients most likely to benefit from third-line TKI therapy represents an important unmet need. A cohort of 26 patients with CP-CML who had failed imatinib and a second-line TKI was analysed to identify prognostic factors for response and outcomes.21 For the achievement of complete cytogenetic responses on third-line therapy, prior cytogenetic response with imatinib or a second-line therapy were the only independent predictors. For OS, younger age and the demonstration of a cytogenetic response on second-line therapy were the only independent predictors. The authors highlighted the need to be able to select more accurately

the patients who would be likely to benefit from third-line TKI therapy. It should be noted however, that the small size of the cohort used may have been inadequate to identify independent risk factors. Median failure-free survival of CP-CML patients receiving third-line therapy is 20 months and this falls to 3–5 months in those with advanced disease.22
In a phase I/II study of 118 patients with CP-CML who had been pretreated with imatinib followed by dasatinib and/or nilotinib, major cytogenetic response was attained by 32% of patients, with a median follow-up of 28.5 months.10 After a 48-month follow-up, major cytogenetic response was newly attained or maintained from baseline by 33% and 7% of patients, respectively.20 Treatment with dasatinib or nilotinib in patients with CML (n=25) previously treated with imatinib and a second TKI were evaluated in a single centre study.19 Of the patients with CP-CML (n=18), 89% achieved a complete haematological response, 13% achieved a complete cytogenic response and 24% achieved a major molecular response. Fifty-six per cent of CP patients lost haematological response with a median of 23 months. In other studies of CP-CML patients who received nilotinib or dasatinib as third-line, responses to treatment were reported and although these were not generally durable, such treatment did appear able to prolong OS in some patients.18,22 The comparative efficacy and optimal sequencing of TKIs as third-line therapies requires prospective evaluation in randomised studies.
Allo-HSCT may be an effective option for treating CP-CML after failure of at least two TKIs.23 In a prospective, randomised study in 669 patients with newly diagnosed CML, 427 were considered eligible for HSCT and were randomised according to the availability of a matched family donor between primary HSCT (166 patients) and best available drug treatment (261 patients). Survival probabilities were not significantly different between the two groups. Patients with a low transplant risk showed improved survival compared with those with high risk (p<0.001) transplant and non-high-risk disease.24 This suggests that HSCT remains a valid option in patients at low transplant risk who have failed TKI treatment. Indeed, progress in transplantation has been made but despite this alloHSCT is associated with 25–30% mortality.25 Further, allo-HSCT is only an option for patients with a good performance status and a suitable stem cell donor. However, no TKI was specifically recommended in the National Comprehensive Cancer Network (NCCN) guidelines for CML treatment after first- and second-line TKI failure and it was noted that newer pharmacological treatments were needed to provide additional options for patients in the third-line therapy setting.
Sub-analysis of the phase I/II study of once-daily bosutinib 500 mg included 118 patients with CP-CML who had been pre-treated with imatinib followed by dasatinib and/or nilotinib, with a median followup of 28.5 months.10 Major cytogenetic response was attained by 32% of patients and a complete cytogenetic response by 24%. Complete haematological response was either achieved or maintained in 73%. Kaplan–Meir-estimated progression free survival was 73% and estimated OS was 84%. Efficacy was also demonstrated at 48-month follow-up.20 Non-haematological treatment-emergent adverse events at 48 months included (all grades; grade 3/4): diarrhoea (83%; 9%), nausea (48%; 1%) and vomiting (38%; 1%); hematologic toxicities included: thrombocytopenia (39%; 26%), neutropenia (21%; 16%) and anaemia (20%; 7%).
Ponatinib (Iclusig®; Ariad Pharmaceuticals, Massachusetts, US) is a novel kinase inhibitor that includes a triple carbon-carbon bond. This bond extends from the purine scaffold and allows the molecule to bind to the ATP-binding pocket without steric hindrance from the bulky isoleucine residue at position 315 of the BCR-ABL mutant T315I.26–28 Ponatinib has been shown to be effective in vitro and in vivo against all clinically relevant BCR-ABL mutations, including the T315I gate keeper mutation,28 a mutation for which no other currently licensed TKI is effective.29 The 12-month data of the PACE (Ponatinib Ph+ ALL and CML Evaluation) pivotal phase II trial showed sustained benefit of ponatinib treatment in heavily pre-treated CML patients. Among the 267 CP-CML patients, 55% had achieved the primary endpoint of a major cytogenetic response (50% of resistant/ intolerant patients and 70% with T315I mutation).27 Based on these results, ponatinib was fast-tracked by the US Food and Drug Administration (FDA) in December 2012 for treatment in patients who could not tolerate therapy with first-line agents or whose disease progressed despite first-line therapy. More recently, with 4 years of follow-up of the PACE trial, ponatinib has been shown to continue to provide benefit to CP-CML patients.30
Among the 267 patients with CP-CML from the 2 years follow-up of the PACE trial, 25% (67 patients) had vascular occlusive events, 7% (20 patients) had cardiovascular serious adverse events, 7% (18 patients) had cerebrovascular serious adverse events, and 5% (14 patients) had peripheral vascular serious adverse events.31 The median time to onset for arterial thrombotic events among the CP-CML patients was 281 (8–952) days. Investigation is underway to identify the mechanisms underlying the vascular toxicity reported with this drug and to explore dose reductions and/or delays required to manage adverse events. The risk of vascular occlusive events may be dose related.32 An integrated dose intensity analysis found that for vascular occlusion, after adjusting for covariates, overall dose intensity is highly statistically significant with an odds ratio of approximately 1.6 for each 15 mg dose increase.32 A dose reduction would therefore be predicted to reduce the risk of vascular occlusive events. The current package insert states that treatment with ponatinib should be reserved for patients carrying the T315I mutation
or who are resistant to dasatinib or nilotinib; intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate.33
In the systematic review of TKIs in CP-CML patients who are resistant/ intolerant to ≥1 prior 2G TKI (bosutinib, dasatinib and nilotinib), the estimated probability of achieving a complete cytogenetic response was 60% for ponatinib compared with 22–26% for the other TKIs. The probability estimated for ponatinib in achieving a higher response rate than the other TKI treatments was 99% for the complete cytogenetic response and 97% for a major cytogenetic response.34 Safety was not compared in this analysis, however.
Guidelines/recommendations on refractory chronic phase chronic myeloid leukaemia management
Guidelines/recommendations for the management of CML patients have been issued by the NCCN (version 1.2016), the European Society of Medical Oncology (ESMO)35 and the European Leukaemia Net (ELN).36 All these organisations have established definitions for haematological, cytogenetic (based on metaphase cytogenetic preparations) and molecular (based on BCR/ABL transcript levels) responses and timelines for achieving various levels of these responses. These evidence-based ‘milestones’ allow close monitoring of the outcome of patients on TKI therapy. Detailed recommendations for treatment of CP-CML patients in first-, second- and third-line from the European LeukemiaNet are
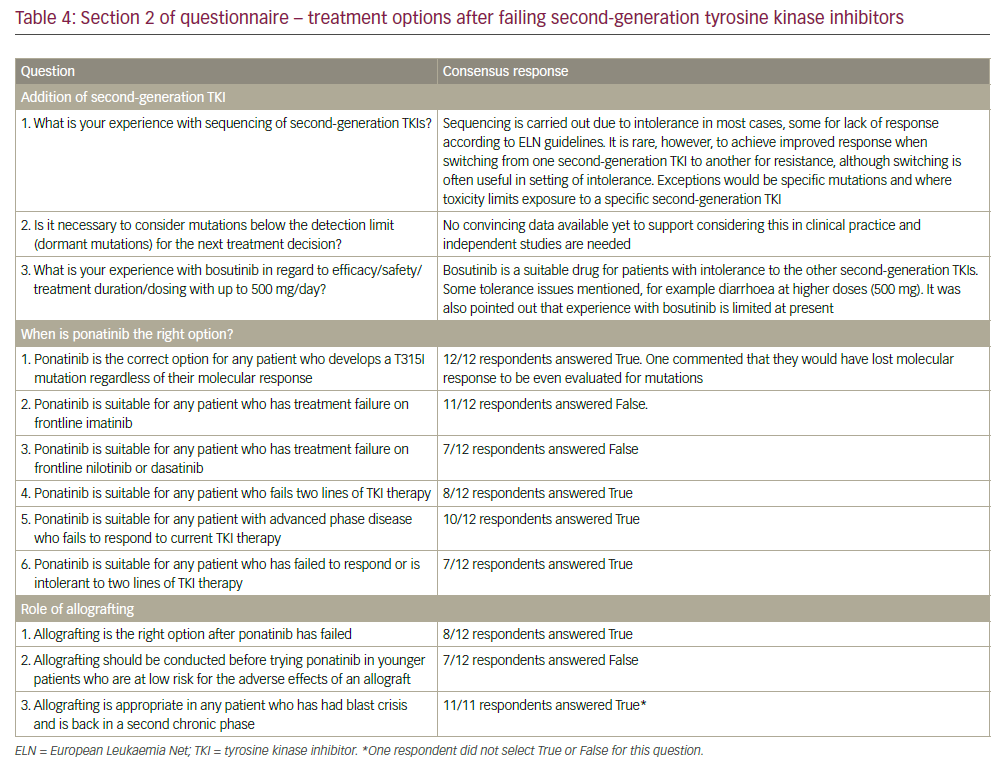
summarized in Table 2. Two elements are in common to all these guidelines/recommendations.
• It is possible to change therapy of imatinib refractory CP-CML patients in second-line or later to an alternate approved TKI (nilotinib, dasatinib or bosutinib).
• HSCT should be considered in case of failure of two TKIs.
A significant difference in the guidelines from the NCCN versus those by the ELN is that not achieving the 10% level at 3 months constitutes a ‘warning’ according to the ELN recommendations, whereas the NCCN takes a stricter approach and defines this as ‘failure’ mandating a change in treatment.
Purpose
The aim of this current paper was to explore the level of consensus, or otherwise, regarding treatment of refractory CP-CML through use of a questionnaire distributed to CML experts across Europe.
Expert opinion questionnaire
Methods
A three-section questionnaire was devised by CML experts in August 2015 to address the following questions:
• What are the effects of pre-treatment?
• What are the treatment options after failing second-generation TKIs?
• How do patients present after failing second-generation TKIs?
CML experts were identified through consultation with the lead authors and the members of the European Oncology & Haematology editorial board. The questionnaire was formatted as an editable PDF and provided to 34 experts by email from September to November 2015, with follow-ups by email as appropriate. To gain a greater number of responses, the questionnaire was then re-sent to a second subset of experts who had not responded in February 2016 (n=9). The questionnaire responses were summarised so the results could be presented in succinct tabular format.
Results
Responses were received from 14 (41.2%) of the persons requested. Two additional questionnaires were incomplete. The geographical distribution of the respondents was: Italy (5), Germany (2), Turkey (1), Spain (1), Netherlands (1), France (2), Russia (1) and Australia (1). The consensus responses of the three sections of the questionnaire are presented in Tables 3–6.
In general, there was a range of opinion on all three areas of question posed. In particular, opinion was divided on the following:
• whether ponatinib is suitable for any patient who has treatment failure on frontline nilotinib or dasatinib (42% agreed with the statement);
• whether ponatinib is suitable for any patient who has failed to respond or is intolerant to two lines of TKIs (67% agreed); and
• whether allografting should be conducted before trying ponatinib in younger patients who are at low risk for the adverse effects of an allograft (42% agreed).
The two questions for which there was a 100% response of ‘true’ were: whether ponatinib was the drug of first choice for any patient who develops a T315I mutation, regardless of their molecular response; and whether allografting is appropriate in any patient who has had blast crises and is back in a second chronic phase. There was also a strong consensus that ponatinib is not suitable for any patient who has treatment failure on frontline imatinib. With 11/12 responses ‘false’ to the question: ‘Ponatinib is suitable for any patient who has treatment failure on frontline imatinib.’ For all the other questions there was a variety of opinions (see Table 4).
Discussion
In this survey, it was highlighted that there may be a higher frequency of the T315I mutation in trial data compared with real-world data. The frequency reported, ranging from around 10% to 27%, may differ because of the various methods and schedules used for mutation testing.37 For example, in the DASISION trial, mutation testing was performed at time of disease progression, treatment failure or end of treatment.38 In the ENESTnd trial, mutation testing was carried out in all patients at baseline (exclusion criteria), and in patients with no baseline mutations, mutation analysis was performed when patients experienced either lack of response or loss of response. And in the PACE trial, the number of T315I mutations accumulated (64/270, 24%) due to efficacy of ponatinib in this setting.39
Ponatinib should not be offered immediately after imatinib, indeed, there is no evidence in support of this and strong agreement on this stance emerged from the questionnaire responses. The only exception are patients who are failing imatinib treatment due to emergence of T315I mutation.
The consensus amongst the experts on the use of ponatinib in any CML patient who was tested positive for the T315I mutation needs to be supplemented with data generated in a retrospective observational study. Transplant is the only cure for resistant- and accelerated-phase CML, and more investigation is needed in this setting for outcomes in those with T315I positive CML. Pooled data from the phase II PACE trial and the European Bone Marrow Transplant registry were used to indirectly compare OS between T315I positive CML patients treated with ponatinib and allogeneic stem cell transplantation (alloSCT). Results showed that CML patients in chronic phase demonstrated significantly longer OS versus CP-CML patients in the alloSCT group: median OS had not been reached in the ponatinib group (hazard ratio [HR] [95% confidence interval (CI)] 0.37 [0.16–0.84]) and was 103.3 in the SCT group (p=0.013). In contrast, there was no significant difference in median OS between the two treatment groups in patients with CML in accelerated phase (HR [95% CI] 0.90 (0.20–4.10); p=0.889) and OS was significantly shorter in patients in blast phase who had received ponatinib versus alloSCT (HR [95% CI], 2.29 [1.08–4.82]; p=0.030). According to these data ponatinib may be a promising alternative for patients with T315I positive CP-CML, but offers no survival advantage for patients in advanced phase of CML.
The response on whether it is necessary to consider dormant mutations for treatment decision-making was that there are inadequate supporting data. Recent data suggested that some mutations may persist at undetectable levels for years after changing therapy and can be reselected, leading to subsequent TKI resistance.40,41 Further, detection of these mutations following imatinib resistance has been recommended to guide subsequent therapy selection. If an inappropriate therapy is selected there is a high risk of treatment failure accompanied by clonal expansion of the resistant mutant.42
All approved BCR-ABL TKIs are effective therapies in CML but are associated with distinct safety profiles, which are not covered in this article. In order to make more tailored treatment decisions clinicians must remain vigilant, recognise low-grade adverse events, and proactively manage adverse events over the long term, considering dose reduction to reduce adverse events and toxicity. In addition, patients are called upon to assess and report subtle changes that might affect their quality of life.
Conclusion
This survey is in no way intended to represent the current state of the art for patients with resistant or refractory disease as it is subject to a number of limitations related to the selection of questions, the knowledge and experience of the experts involved, the willingness to reply in detail, and more. Not all selected experts contributed to the survey so that the response does not represent a broad consensus of opinion but provides a selective snapshot of current opinions. However, this survey with highly discordant opinions on treatment and management of these patients demonstrates the high need for generating more mature data and for more intensive exchange on peer-to-peer level to increase the consensus level across the community.
APPENDIX
The following individuals provided completed questionnaires and inputted on drafts during the development of this manuscript:
• Michele Baccarani (Hematology and Oncology L and A Seragnoli, S Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy)
• Angelo Carella (Hematology Unit, IRCCS San Martino, Genova, Italy)
• Francisco Cervantes (Hematology Department, Hospital Clínic, University of Barcelona, Barcelona, Spain)
• Ahmet Emre Eskazan (Division of Hematology, Department of Internal Medicine, Cerrahpasa Faculty of Medicine, Istanbul University,
Istanbul, Turkey)
• François Guilhot (Department of Oncology, Hematology, and Cell Therapy, Centre Hospitalier Universitaire de Poitiers, Clinical Investigation
Center, Inserm 0802, Poitiers, France)
• Rüdiger Hehlmann (Medizinische Fakultät Mannheim der Universität Heidelberg, Mannheim, Germany)
• Perttu Koskenvesa (Hematology Research Unit Helsinki, Department of Medicine, Helsinki University Central Hospital and University of Helsinki,
Helsinki, Finland)
• Francois-Xavier Mahon (Bergonié Cancer Institute, INSERM Unit 916, University of Bordeaux, Bordeaux, France)
• Giovanni Martinelli (Department of Experimental, Diagnostic and Specialty Medicine (DIMES), Unit of Hematology, University of Bologna,
Bologna, Italy)
• Gianantonio Rosti (Department of Hematology and Oncology ‘L. and A. Seràgnoli’, St Orsola University Hospital, Bologna, Italy)
• Dominik Wolf (University Hospital Bonn [UKB], Bonn, Germany)
• Andrey Zaritskey (Center of Heart Blood and Endocrinology, St Petersburg, Russia)











