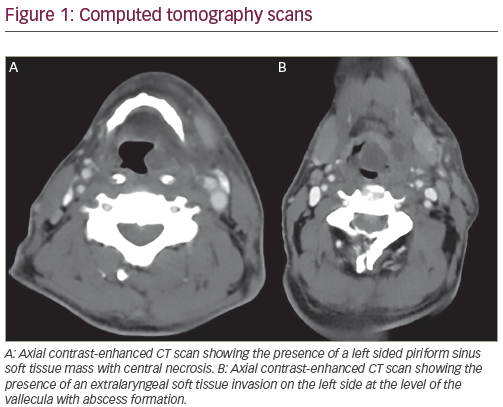
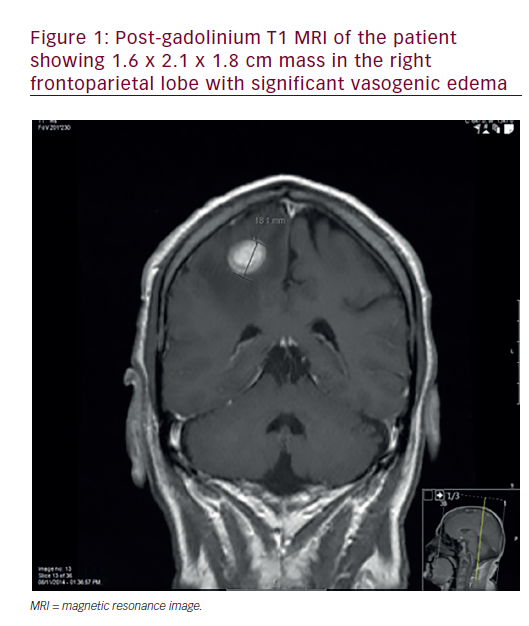
General Considerations
Available treatment options for brain metastasis include focal (i.e. surgery and radiosurgery) and non-focal (whole-brain radiotherapy and chemotherapy) treatment modalities. In spite of numerous randomised trials, the optimal timing and patient selection for each of these treatment modalities remains contentious. This controversy seems to derive from two main issues: the first is related to the extreme heterogeneity of patients with brain metastasis, who can differ considerably in terms of prognostic characteristics such as primary cancer type, systemic disease control, brain metastasis location, number of lesions, age, performance status, and presence of cognitive or other neurologic impairment. This renders extremely difficult the task of extrapolating generic results derived from clinical trials to an individual patient. To address this issue, the Radiation Therapy Oncology Group (RTOG) proposed a classification based on a recursive partitioning analysis (RPA) of a large population of patients with brain metastasis in an effort to homogenise patient populations for clinical trials and facilitate treatment decisions. For this classification, patients are divided into three classes based on Karnofsky performance status (KPS), age and extent of systemic disease. The resulting stratification has prognostic value and has been validated in a variety of primary cancer types. Although the RPA classification may help provide general guidelines, it is not perfect, particularly because it does not take histology into consideration.
A second major source of controversy in the management of brain metastasis has been the lack of trials adequately designed and powered to investigate questions related to the balance between successful tumour control and long-term treatment-related neurocognitive impairment. Radiotherapy is particularly associated with an increased risk of neurotoxicity; however, it has been difficult to ascertain the magnitude of this problem, especially because it is difficult to differentiate tumour burden on neurologic function from neurotoxic effects. Moreover, assessment of neurotoxicity depends on long-term neuropsychological follow-up, which has been difficult to incorporate into large prospective studies. Results of available clinical trials and details pertaining to each treatment modality are reviewed below.
Whole-bra in Radiation Therapy
Whole-brain radiation therapy (WBRT) has historically been the most important modality of treatment for brain metastases. Phase III trials have demonstrated that WBRT achieves radiographic responses and improves neurologic function in approximately 50% of patients; median survival increases to 4 6 months. Central nervous system (CNS) disease is the cause of death in approximately half of these patients, while the other half will die from systemic disease progression. Multiple trials have tried to determine the optimal dose and schedule but to date there is little evidence to support that hyperfractionated schedules or higher doses are superior to the traditional dose of 30Gy fractionated in 10 daily sessions. WBRT has the advantage of treating microscopic disease and is consensually indicated for patients with multiple (more than three) lesions, since these are not good candidates for focal treatment. However, the use of WBRT in potential candidates for focal therapies has been controversial. There is strong evidence to support that WBRT improves local control when added to focal therapies; but a major concern is the risk of development of late-delayed neurotoxicity among long-term survivors. The incidence of such a complication has been estimated at 10% to 20%, with elderly patients at particular risk. Common symptoms of neurotoxicity are dementia, gait ataxia, and incontinence; quality of life is profoundly affected in the presence of such a complication. Therefore, many authors recommend starting with focal modalities of treatment or reducing the dose per fraction in patients with life expectancy greater than nine months. However, others consider that the burden of tumour recurrence on neurologic dysfunction when WBRT is withheld surpasses the risk of neurotoxicity and that WBRT should therefore be indicated for all patients. In the lack of adequately powered trials incorporating neuropsychological end-points and long-term followup, the decision of indicating WBRT for RPA class I patients should be taken on an individual basis. In any case, WBRT remains a widely accepted option for a majority of RPA class II and III patients, since they will typically die before developing neurotoxicity.
The addition of radiosensitisors is an emerging strategy of treatment for improving the efficacy of WBRT. After several negative trials utilising a variety of new radiosensitisors, a recent phase III study comparing WBRT with and without motexafin gadolinium (MGd) in RPA class I and II patients has suggested that this drug may benefit patients with lung cancer in terms of time to neurologic progression. However, survival benefit was not seen in any histology and, to date, US Food and Drug Administration (FDA) approval has not been granted to this drug. More importantly, that trial has demonstrated the feasibility of incorporating neurocognitive outcomes in the design, which in fact can be even more relevant than survival end-points in such population; moreover, these results suggested that different histologies should be studied separately. Other trials on radiosensitisors are under way.
Surgery
brain metastases has also been extensively debated. Well-accepted indications for surgery include lesions with extensive mass effects that need to be evacuated, and the necessity of obtaining tissue for diagnostic confirmation. Other indications for surgery are more controversial. As demonstrated in two randomised trials, surgical resection for the treatment of single lesions prior to WBRT achieves longer median survival and functional independence than WBRT alone. Patients younger than 65 years, with a KPS of more than 70 and controlled systemic disease, seem to benefit most. A third trial did not find any differences but their population included more patients with a lower KPS and active systemic disease. Taken together, these results suggest that surgery is a suitable option for patients with a single resectable lesion whose systemic disease is under control. The decision to add WBRT for these patients should be individualised and should follow the principles described above. One study comparing surgery alone versus surgery plus WBRT demonstrated that tumour recurrence and death due to neurological causes was lower in the group subsequently treated with WBRT, validating the concept that WBRT improves local control. However, overall survival and duration of functional independence was similar in both groups, reflecting the morbidity and mortality related to systemic disease progression even when local control is achieved. These results have been interpreted in both ways, particularly because there is no sufficient data on the development of neurotoxicity in long-term survivors. Focal external beam radiation to surgical bed is another strategy under investigation for improving local control after surgery and might be a useful tool particularly for those patients in whom surgical resection was incomplete. There are no randomised trials looking at the role of surgery in patients with more than one lesion. Retrospective series from centres with good surgical experience have suggested that resection of up to three lesions in selected patients is feasible, safe and may yield results similar to patients with single lesions. However, such an approach should be limited to selected patients, particularly for those situations when surgery is necessary to decrease mass effect.
Stereotatic Radiosurgery
Stereotatic radiosurgery (SRS) is a technique for delivering highly focal external irradiation to a clearly defined small target, allowing the use of high doses of radiation without damaging adjacent normal tissue. Gamma-rays (gamma knife), high energy X-rays (linear accelerator) and proton beams are different techniques utilised that seem to achieve comparable results. SRS is a relatively non-invasive method, does not require hospitalisation and allows the treatment of surgically inaccessible lesions. It is also effective for those tumours known to be relatively radioresistant such as melanoma, renal cell carcinoma and sarcoma. The main limitation is that it can only be used for treating lesions under 4cm in diameter. Late complications occur in 10% of patients, including symptomatic radionecrosis that requires treatment with steroids and, rarely, surgical resection. As with surgery, there has been some controversy over the role of adding SRS to WBRT. A prospective phase III trial conducted by the RTOG enrolled 333 patients with one to three newly diagnosed brain metastases to receive WBRT either with or without SRS. Although a survival benefit was clearly demonstrated only in patients with a single lesion (median overall survival (OS) of 6.5 versus 4.9 months; p=0.03), patients in the WBRT+SRS arm were significantly more likely to have a stable or improved KPS compared with the WBRT alone group (stable or improved KPS at six months of 43% versus 27%, respectively). RPA class 1 and favourable histological status were predictors of survival on multivariate analysis. These results suggest that the addition of SRS to WBRT seems to be beneficial to all patients who are candidates for SRS. However, whether SRS can substitute WBRT as initial treatment in this population remains unclear, since no prospective studies are available. One retrospective study with 569 patients with newly diagnosed metastases demonstrated that SRS alone provided a survival similar to SRS plus WBRT, suggesting that focal treatment without WBRT could be a reasonable initial approach in selected patients. The role of SRS compared with surgery is even less clear. The RTOG is conducting a randomised prospective trial directly comparing these treatment modalities, but accrual has been very slow due to a strong preference of patients for one modality or the other. Retrospective experience has demonstrated no major differences between these two modalities, but available studies have several limitations. Therefore, to date, SRS should be seen as an alternative to surgery in those patients most likely to benefit from focal control, particularly RPA class I patients who have controlled systemic disease and up to three lesions; it would be the procedure of choice over surgery for those with lesions in surgically inaccessible areas or those with other contraindications to surgery.
Chemotherapy
The role of chemotherapy in treating brain metastasis is limited, and response seems to vary according to the type of primary tumour. The rationale for its use would be the possibility of treating both primary tumour and metastases. However, many patients with brain metastasis have already failed first-line chemotherapy and have limited therapeutic options; moreover, it has been observed that brain lesions respond less frequently to chemotherapy compared with the primary tumour. This seems to be explained by intrinsic properties of the metastatic lesion, conferring chemoresistance, as well as the presence of the BBB. It is accepted that the BBB is disrupted in the metastatic lesion. However, it is difficult to assess whether this is enough to allow water-soluble agents to achieve therapeutic concentrations within the affected region. Nevertheless, there are examples of water-soluble agents causing regression of brain metastases, and selecting agents known to have activity against the primary cancer type is key.
Metastases resulting from certain types of chemosensitive tumours are more likely to respond to chemotherapy, particularly SCLC, but also testicular, NSCLC, breast and melanoma. Chemo-naïve and RPA class I patients also seem to exhibit a better response to chemotherapy. For example, response rates for newly diagnosed, untreated SCLC seem to be around 70% to 80% with chemotherapy alone, while for previously treated patients the response is 40%. Therefore, chemotherapy may be considered the first line of treatment for chemo-naïve SCLC patients. For all other situations, WBRT or focal therapies are considered standard treatment and chemotherapy should be reserved as a salvage strategy in the event of recurrence.
Recurrent Brain Metastasis
The treatment of recurrences should take into consideration the present status of both systemic and CNS disease, as well as previous treatments. If the patient has been treated with focal modalities, and continues to have controlled systemic disease, the first step would be to assess whether the patient is still a candidate for focal therapy. Surgery would be indicated for those with extensive mass effect and could be considered for palliation. Lesions previously resected may benefit from radiosurgery. If these options are not feasible or if the patient has progressive systemic disease, WBRT should be considered. Re-irradiation with WBRT may be used with palliative intent, particularly if prior treatment occurred more than one year earlier. Chemotherapy is an option to be considered for chemosensitive tumours, with drugs appropriate for those types of tumours. Two studies using temozolomide for recurrent metastasis from solid tumours have demonstrated some efficacy and it may be an interesting option, particularly for patients with NSCLC. Other drugs are being tested as single agents or in combination.
Conclusions
Successful treatment of brain metastasis relies on the adequate control of CNS disease as well as systemic tumours. Despite major advances in strategies for focal control, patients continue to die, either from brain recurrence or systemic disease progression. Ongoing clinical trials will help to optimise the use of available treatment modalities but the key seems to be the development of new treatment strategies that address both systemic and CNS disease; the mechanisms of chemoresistance in brain metastases need to be further clarified. For the time being, patients and family should be made aware of potential risks and benefits of available treatment options.