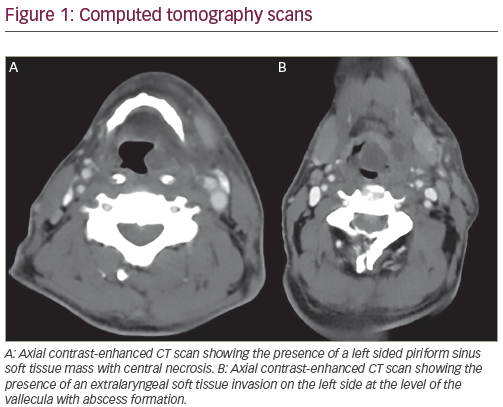
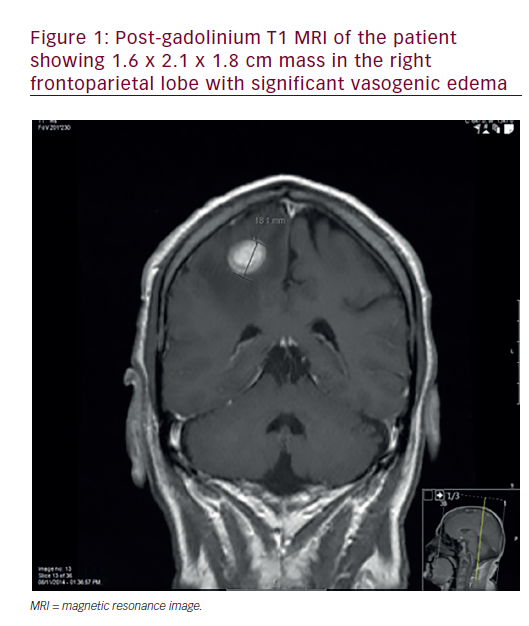
Oesophageal adenocarcinoma is currently the most rapidly increasing cancer in the US and Western Europe.1,2 This type of tumour is frequently detected at an advanced stage and requires multimodal treatment. Despite improvements in its detection, surgical resection and neoadjuvant therapy, the overall survival of oesophageal cancer remains lower than that of other solid tumours.3 In fact, a recent meta-analysis by Greer et al., analysing 21 randomised trials of neoadjuvant radiochemotherapy prior to surgery for patients with oesophageal cancer, showed a non-significant improvement in overall survival.4 In the last few years, there has been an exponential growth in our understanding of the cellular and molecular events associated with cell-cycle regulation, programmed cell death, angiogenesis and tumour growth. The discovery and characterisation of growth factor receptors – agents that stimulate or inhibit angiogenesis, cell-cycle regulatory proteins and other compounds that regulate cellular function – led to the possibility that biologic therapy could be used to prevent or treat neoplasia. Oncologists are currently investigating several novel targets, both as single agents or in combination with chemotherapy, to assess the potential for increased efficacy. Pre-clinical and clinical studies have described potential tumour targets in oesophageal cancer, including cyclo-oxgenase-2 (COX-2), epidermal growth factor receptor (EGFR) and vascular endolethial growth factor (VEGF).5 The goal of this article is to provide an update of the most recent data of targeted therapy against these three factors in oesophageal cancer.
Cyclo-oxygenase-2
COX-2 and the isoform COX-1 are rate-limiting enzymes in the conversion of arachidonic acid to prostaglandins. It is well-known that COX-2 participates in the regulation of a broad range of cellular processes, including proliferation, angiogenesis and resistance to apoptosis. Pre-clinical studies have shown that COX-2 is sequentially increased in the metaplastic–dysplastic sequence leading to oesophageal adenocarcinoma.6–8 In addition, epidemiological studies have revealed that regular Aspirin® use is associated with a decreased risk of oesophageal cancer, an effect that was also observed with selective COX-2 inhibitors in a case-control study.9,10 These findings implied that the inhibition of COX-2 is an effective strategy in the prevention and treatment of oesophageal adenocarcinoma. In fact, preliminary data from several phase I/II trials assessing the potential of combining COX-2 inhibitors and concurrent chemoradiotherapy for locally advanced oesophageal cancer are already available. In a phase II trial, Enziger et al. analysed the efficacy of neoadjuvant therapy with cisplatin, irinotecan, concurrent radiation therapy and celecoxib, a selective COX-2 inhibitor, in patients with locally advanced oesophageal cancer.11 In their preliminary analysis, 25 out of 36 patients had completed chemoradiation and surgery, of whom 11 had a complete pathological response. Govindan et al. reported the first results of a phase II study conducted by the Hoosier Oncology Group (HOG) in patients with potentially resectable oesophageal cancer receiving cisplatin, 5-fluorouracil, celecoxib and radiation therapy.12 Of the 31 patients included in the study, five had a complete pathological response. In a clinical study by Altorki et al., an overall response rate of 57% was observed in patients with oesophageal cancer receiving pre-operative paclitaxel/carboplatin and celecoxib followed by adjuvant celecoxib.13
Finally, Dawson et al. demonstrated in a phase I/II trial of celecoxib with chemotherapy and radiotherapy in the treatment of patients with locally advanced oesophageal cancer that the regimen was well tolerated, with a radiological response rate of 54%.14 The results of these pilot studies are encouraging; however, further follow-up is necessary to determine the continued safety of celecoxib and its impact on disease progression and overall survival in patients with oesophageal cancer.
Epidermal Growth Factor Receptor
EGFR is a member of the type I receptor tyrosine kinase family. It has been correlated with various cellular processes involved in carcinogenesis, such as cell proliferation, inhibition of apoptosis, angiogenesis, cell motility and metastasis.15,16 In oesophageal cancer, overexpression of EGFR occurs in 30–90% of cases and correlates with poor prognosis, suggesting that targeting EGFR is a valid strategy in the treatment of this tumour type.17 In fact, pre-clinical studies have shown that anti-EGFR agents are able to inhibit these EGFR signal transduction pathways and additionally potentiate the effectiveness of traditional anticancer therapy.15 There are four strategies for inhibiting EGFR:18–20
• EGFR-specific monoclonal antibodies bind to the domain of the receptor and competitively inhibit ligand binding;
• receptor tyrosine kinase inhibitors compete with adenosine triphosphate (ATP) for the intracellular catalytic site of EGFR and thereby interrupt the downstream cascade that would normally be activated by a ligand binding to EGFR;
• antisense oligonucleotides are directed against EGFR messenger RNA (mRNA), resulting in the inhibition of proliferation and induction of apoptosis; and
• EGFR ligand–toxin and immunotoxin conjugates contain an EGFR-binding antibody or an EGFR ligand that is conjugated to a potent cellular toxin.
In gastrointestinal tumours, including oesophageal cancer, the first two strategies are the most developed; recent clinical studies of them are discussed below.
Monoclonal Antibodies Targeting Epidermal Growth Factor Receptor
To date, the most promising monoclonal antibody targeting EGFR is cetuximab (Erbitux®). Over the last few years, several clinical studies have shown promising efficacy of cetuximab in patients with colorectal cancer, leading to the approval of the use of cetuximab in patients with metastatic colorectal cancer by the US Food and Drug administration (FDA).21–23
However, fewer data are available assessing the efficacy of cetuximab in oesophageal cancer. Suntharalingam et al. recently presented preliminary data from an ongoing clinical trial evaluating the safety and efficacy of the addition of cetuximab to concurrent chemoradiation for patients with oesophageal and gastric cancer.24 While cetuximab was administered safely and without major toxic effects, in 18 of 27 patients they detected a clinically complete response and in seven of 16 patients who underwent surgery they detected a pathologically complete response. In a phase II study by Enzinger et al. assessing the safety and efficacy of cisplatin, irinotecan, cetuximab and concurrent radiation therapy followed by surgery in patients with locally advanced oesophageal cancer, early results suggest a lower complete response rate and higher overall toxicity compared with other available therapeutic regimens.25 Further evaluation of these studies (including those in progress) evaluating the efficacy of cetuximab in patients with oesophageal cancer is needed.26
Antiepidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors are orally available small molecules. They inhibit cellular tyrosine autophosphorylation, resulting in a blockade of EGFR signal transduction pathways. Pre-clinical studies have described the blockade of tyrosine kinase activity, pro-apoptotic effects and inhibition of cell proliferation and angiogenesis as mechanisms of action of tyrosine kinase inhibitors.18,20 Several clinical trials, especially in patients with non-small-cell lung cancer, indicate a good efficacy of tyrosine kinase inhibitors in cancer treatment.27,28 Currently, clinical data describing the efficacy of anti-EGFR tyrosine kinase inhibitors in patients with oesophageal cancer are sparse.
Tew et al. analysed the safety and efficacy of erlotonib, an anti-EGFR tyrosine kinase inhibitor, in a phase II trial for second-line treatment in advanced oesophageal cancer.29 They demonstrated that erlotonib is an active and well tolerated treatment for advanced oesophageal cancer, with disease control rate in 11 of 20 patients and partial response in three. Initial results from three other clinical trials investigating the efficacy of anti-EGFR tyrosine kinase inhibitors in oesophageal cancer were shown to be welltolerated, with a modest response rate.30–32
Vascular Endolethial Growth Factor
VEGF has been shown to be the most potent angiogenic factor and a powerful mediator of tumour angiogenesis.33 The importance of its role in carcinogenesis is reflected by its association with the metastatic potential and prognosis of gastrointestinal cancers.34 Evidence of its oncogenetic role is the correlation of tumour microvessel density with VEGF expression.36 Of interest, the goblet cells in Barrett’s mucosa stain intensely for VEGF, suggesting that they may supply the factor on the pathway leading to cancer.36 Recognition of VEGF as a key regulator of angiogenesis has led to considerable interest and efforts to exploit its potential for anticancer therapy. A great variety of strategies targeting VEGF or its receptor signalling system are currently being developed for cancer therapy. They are promising, and the most advanced in clinical development is bevacizumab (Avastin®), a recombinant humanised monoclonal antibody against VEGF.33 In fact, bevacizumab combined with irinotecan-based chemotherapy significantly improved overall survival in patients with metastatic colon cancer, leading to its FDA approval.37 To date, clinical evidence showing an efficacy of bevacizumab treatment in oesophageal cancer is sparse. There is only one multicentre phase II study of irinotecan, cisplatin and bevacizumab in patients with unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma.38 In 16 patients with measurable disease, Shah et al. observed 12 confirmed partial responses, three minor responses and one stable disease without an increase in irinotecan/cisplatin-related toxicity.
Nevertheless, thromboembolic events were seen in 25% of patients: four patients with asymptomatic pulmonary embolism and two patients with deep vein thrombosis. Therefore, further clinical trials, including those ongoing in the US, need to assess the safety and efficacy of bevacizumab in the anticancer therapy of patients with oesophageal cancer.39
Conclusion
Although targeted therapy in oesophageal cancer is in early development, promising results have already been reported from clinical trials assessing the efficacy of this novel treatment option. Results of further clinical studies are needed to establish targeted agents in the treatment of oesophageal cancer. Identifying predictive markers for this anticancer treatment to increase complete pathological response rate in patients treated with neoadjuvant chemotherapy must be a goal of research. This will identify prognostic markers to select patients for chemotherapy who are at high risk of tumour recurrence after a successful resection. It will also help to tailor chemotherapy, making it not only more effective, but also less toxic. ■
My Learning
Login
Sign Up FREE
Register Register
Login
Trending Topic

12 mins
Trending Topic
Developed by Touch
Mark CompleteCompleted
BookmarkBookmarked
Allan A Lima Pereira, Gabriel Lenz, Tiago Biachi de Castria
NEW
Despite being considered a rare type of malignancy, constituting only 3% of all gastrointestinal cancers, the incidence of biliary tract cancers (BTCs) has been increasing worldwide in recent years, with about 20,000 new cases annually only in the USA.1–3 These cancers arise from the biliary epithelium of the small ducts in the periphery of the liver […]
touchREVIEWS in Oncology & Haematology. 2025;21(1):Online ahead of journal publication