Ocular melanoma represents a small subset of total melanoma cases. Approximately 3.7% of melanoma cases are ocular,1 and slightly over 80% of ocular melanomas are classified as uveal.1,2 Most uveal melanomas occur from the choroid, with the remainder developing from the ciliary body or iris.1 The rate of ocular melanoma is 8–10 times higher in white than black people, and it is slightly more common in men than women.1
The overall 5-year survival of uveal melanoma has been reported as high as 82%;2 however, survival drops off significantly once metastatic disease is present. The Collaborative Ocular Melanoma Study (COMS) Group found that the rates of metastatic disease at 5 and 10 years after diagnosis were 25% and 34%, respectively.3 The most common site of metastasis is the liver, with liver lesions present in 77–94% of patients with metastatic disease.3–6 Other common sites of metastasis include lung and bone.3,6 Once uveal melanoma metastasizes, the median survival is only 2 months without treatment.5 Even despite treatment, the median survival is still typically less than one year.3–5,7
The disheartening survival rates in metastatic disease underscore the importance of developing treatments for metastatic uveal melanoma. Chemotherapy regimens, similar to those used with cutaneous melanoma, have demonstrated dismal response rates between 0–6%, and none have been shown to prolong overall survival.8–12 Because the liver is frequently the sole site of metastatic disease, hepatic-directed therapy is often considered. Advances in molecular biology have led to an understanding of distinct genetic differences between uveal and cutaneous melanoma, which highlight the need for distinct treatments. This review describes the current therapeutic options in the treatment of metastatic uveal melanoma.
Molecular biology
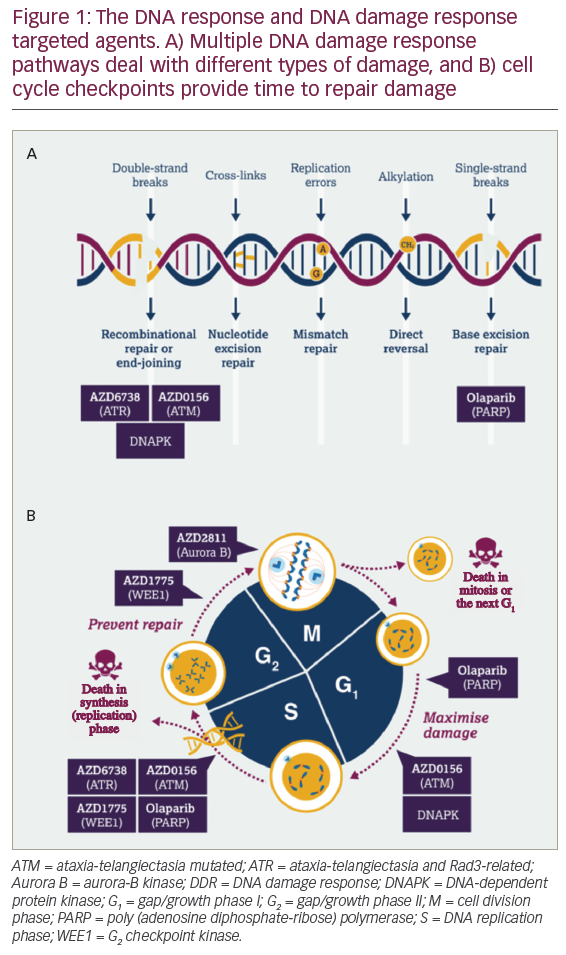
The mitogen activated protein kinase (MAPK) pathway plays a significant role in cutaneous and uveal melanoma through its effects on cell proliferation. In cutaneous melanoma, upstream mutations in BRAF and NRAS activate the MAPK pathway and affect cell division and apoptosis.13,14 BRAF and NRAS mutations are not typically found in uveal melanoma, but the MAPK pathway is constitutively activated nonetheless.15 Instead, the GNAQ and GNA11 genes encode the alpha subunit of G-proteins that become proto-oncogenes when mutated. These mutations activate the MAPK pathway, leading to uninhibited cell proliferation and contributing to the development of uveal melanoma.16,17 Mutually exclusive mutations in GNAQ or GNA11 are seen in 83% of uveal melanomas.15 The various signaling pathways that are potential therapeutic targets in uveal melanoma include the MAPK, protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathways.18–22
Receptor tyrosine kinases c-kit and c-Met (MET) have also been implicated in the pathogenesis of uveal melanoma, promoting tumor development and metastasis.23–25 Overexpression of the chaperone protein heat shock protein 90 (Hsp90) alters the Akt pathway to inhibit cell death, and its presence is associated with a worse prognosis.26 Loss of the tumor suppressor BRCA Associated Protein 1 (BAP1) also portends a poor prognosis and is highly associated with metastatic disease.27
Hepatic-directed therapy
In contrast to cutaneous disease, the development of metastases in uveal melanoma often follows a predictable pattern with the liver as the most common site of tumor spread.3–6 In many patients with metastatic disease, the liver is the only site of metastatic disease and is often the driver of morbidity and mortality.4,28
Surgery
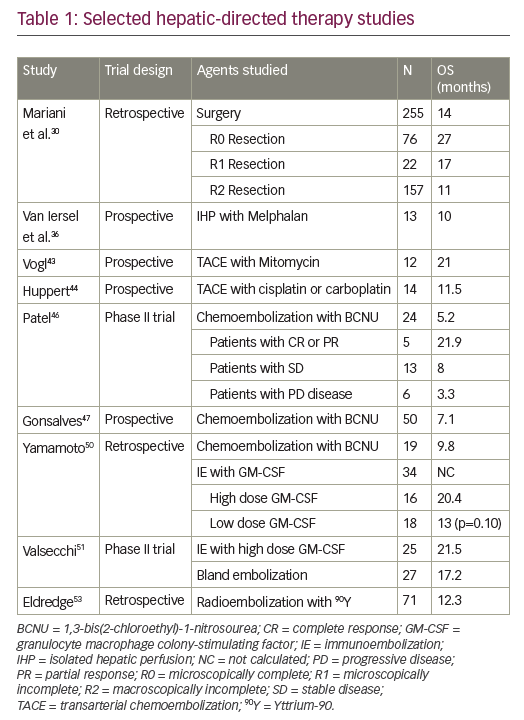
Patients with limited sites of disease should undergo evaluation by a multi-disciplinary team for consideration of metastasectomy. Surgical resection of liver lesions has been shown to improve overall survival in several observational studies in highly selected patients. A major limitation however, is that the vast majority of patients with metastatic uveal melanoma will not qualify for surgical resection due to the number or distribution of lesions.29 In one retrospective study including 255 patients who underwent surgical resection, the median overall survival was 14 months.30 Survival directly correlated with the surgical margin status. In patients who had an R0 resection, the overall survival was 27 months.30 Of the few patients who qualify for surgery, only 30–37% of them will undergo an R0 resection.30,31 Other prognostic factors to consider include the number of lesions present in the liver, with a smaller number of lesions predicting a better response to surgical resection.28,30,31
Radiofrequency ablation
Because many patients with liver metastases will not qualify for surgical resection, radiofrequency ablation (RFA) has been investigated as an alternative option for poor surgical candidates with a small number of liver lesions. A retrospective study of 20 patients with liver metastases from melanoma found that overall survival in these patients was 19.3 months after treatment with RFA; however, patients with six or more metastatic liver lesions were excluded from the study.32 RFA may be a potential therapeutic option in the future, but further studies are needed to determine efficacy of this treatment.
Direct therapy
The dual blood supply to the liver presents a unique opportunity for delivering therapy directly to liver metastases since liver tumors are almost exclusively supplied by the hepatic artery.33 Various techniques utilize the dual blood supply of the liver to preferentially deliver chemotherapy, radiotherapy, or immune therapy to the tumors while minimizing effects on the normal hepatic parenchyma. Large doses of chemotherapy can be delivered through the hepatic artery to achieve high concentrations in the liver while mitigating systemic side effects. Direct therapies have not prolonged overall survival but have shown modest benefit in selected subsets of patients. Future studies are needed before these therapies can be universally recommended.
Hepatic perfusion
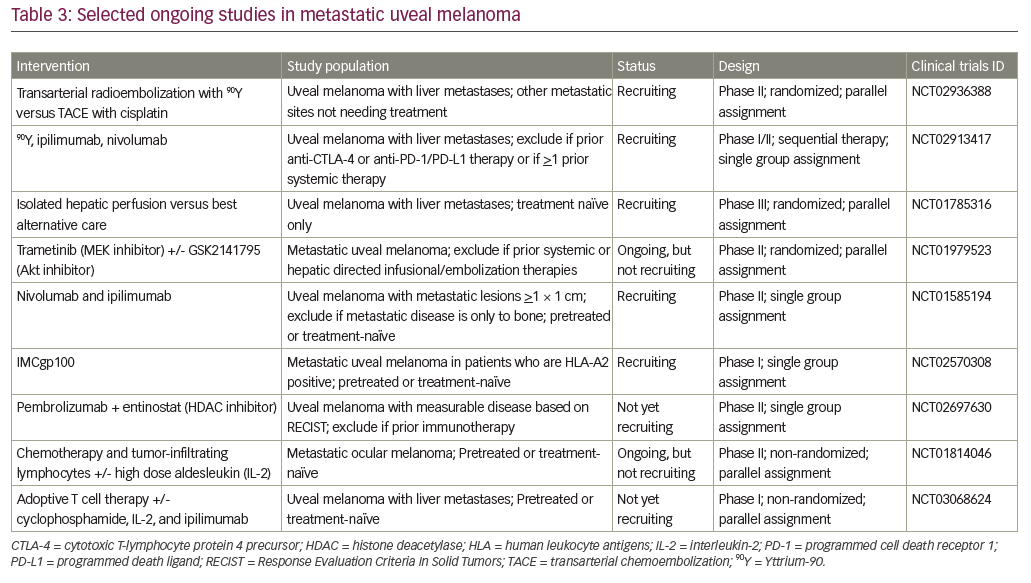
The delivery of melphalan chloride to the liver via a surgical or a percutaneous approach has also demonstrated some potential for disease control. Isolated hepatic perfusion (IHP) is an open surgical technique that allows chemotherapy to be delivered to the liver while it is temporarily sequestered from the systemic circulation. The surgeon isolates the liver from the systemic circulation by clamping inflow arteries and outflow veins. Once the liver is isolated, high doses of chemotherapy are delivered to the liver with an extracorporeal perfusion circuit.34,35 IHP is associated with high response rates, ranging from 33–68%,36–40 and a potential survival benefit of up to a year.37 Despite encouraging overall survival rates,36,38,40 this technique is not widely accepted due to lengthy procedure times, long hospitalizations,27 the morbidity associated with the procedure,36,40 and its inability to be repeated.35,40 A phase III trial comparing IHP to best alternative care is ongoing (NCT01785316).
For patients who are poor surgical candidates or who may need repeated procedures, a percutaneous alternative to IHP is available. Percutaneous hepatic perfusion (PHP) utilizes a double-balloon catheter to block the hepatic venous outflow while melphalan is delivered through a catheter in the hepatic artery. In both IHP and PHP, the systemic circulation is maintained through a venoveno bypass circuit while the procedure is taking place. Phase I trials demonstrated the safety and feasibility of PHP.41 A recent phase III trial that compared PHP to best alternative care found statistically significant differences in hepatic progression free survival (7.0 versus 1.6 months), overall progression free survival (5.4 versus 1.6 months), and hepatic objective response (36.4% versus 2.0%). The advantage in overall survival did not reach statistical significance possibly due to the high crossover rate.42 A second phase III trial comparing PHP to one of four best alternative care options is currently recruiting participants (NCT02678572).
Chemoembolization
Transarterial chemoembolization (TACE) delivers high concentrations of chemotherapy to the liver while also restricting blood supply and inducing ischemia of the tumor. The procedure is well tolerated, decreases systemic side effects, limits washout of chemotherapy, and can be repeated multiple times in the same patient.43,44 Favorable clinical benefit rates have been shown in small studies.44,45 Typically, patients with less liver involvement have a greater survival benefit.44–46 Once liver involvement is greater than 50%, no difference in survival is seen.47 Patients with nodular disease had longer progression free survival and overall survival compared to those with diffuse liver involvement.48 At present, only limited data are available regarding optimal hepatic directed therapies, a clinical trial is currently recruiting participants to compare TACE to radioembolization in patients with metastatic uveal melanoma (NCT02936388).
Immunoembolization
Like chemoembolization, immunoembolization (IE) can deliver treatment preferentially to liver metastases via the hepatic artery. Instead of delivering chemotherapy, IE delivers granulocyte macrophage colony-stimulating factor (GM-CSF) to stimulate the immune system to target tumor specific antigens. A phase I clinical trial in patients with uveal melanoma found a 32% radiographic response rate with immunotherapy, and a higher GM-CSF dose (>1500 ug) was associated with prolonged survival.49 A retrospective study of 53 patients showed that high dose IE resulted in longer overall survival than chemoembolization.50 In the subset of patients with >20–50% of liver involvement by tumor, IE is favored over bland embolization as well.51,52 No randomized trials comparing chemoembolization and IE have been completed, and IE is not widely performed outside of selected centers.
Radioembolization
Radioembolization can utilize the liver’s dual blood supply to deliver radiation preferentially to liver metastases. A beta-emitting isotope Yttrium-90 (90Y) is encapsulated in microspheres with an average penetration of 90Y is 2.5 mm, so radiation induced liver injury is uncommon. 90Y has been used in metastatic disease to the liver for many years, but no clinical trials involving uveal melanoma have been completed. Retrospective studies of metastatic uveal melanoma treated with radioembolization have demonstrated median overall survival rates from 7 months to over 1 year after treatment.53–55 Further data is needed before radioembolization can be widely adopted, and a phase I/II study to evaluate for synergy between radioembolization and immunotherapy is currently underway (NCT02913417). See Table 1 for selected hepatic-directed therapy studies.
Systemic therapy
Contrary to hepatic-directed treatments, systemic therapies have the ability to treat tumor cells throughout the body, including microscopic disease. Conventional systemic chemotherapy, however, has shown poor response rates in uveal melanoma.8–12 A number of genes and intracellular signaling pathways play important roles in the pathogenesis of uveal melanoma, the development of metastases, and the aggressive nature of this cancer. An increased understanding of the molecular genetics and intracellular signaling of uveal melanoma has led to the development of immunotherapy and targeted systemic therapies.
CTLA inhibition
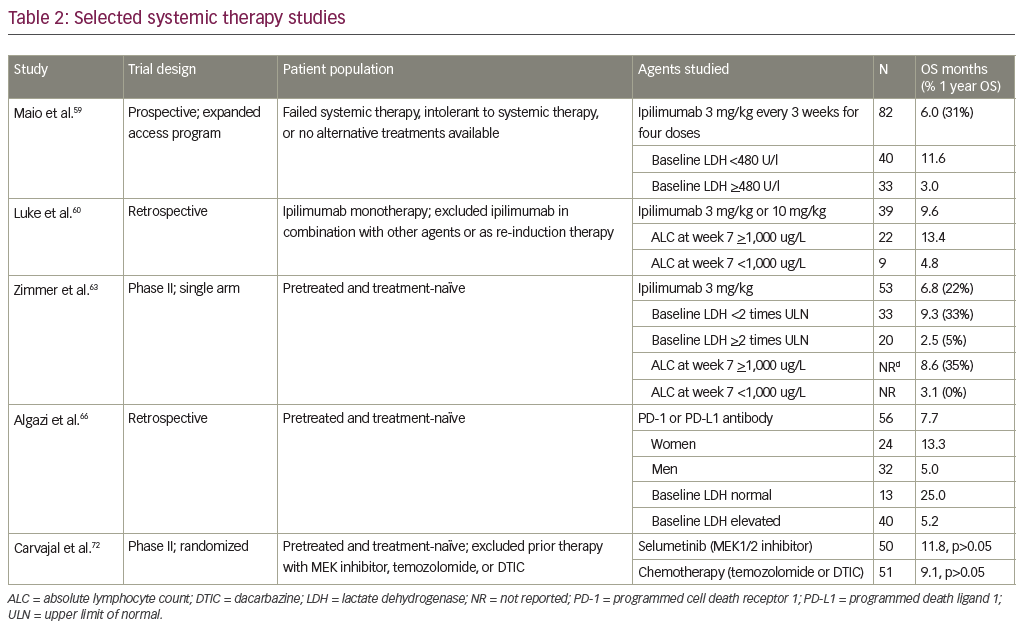
Targeting the cytotoxic T lymphocyte associated antigen-4 (CTLA-4) receptor by ipilimumab is a treatment option for patients with metastatic cutaneous melanoma. A pooled analysis of survival data from 1,861 patients revealed 22% of patients survived to at least 3 years, at which time the survival curve plateaued. In some studies patients were followed almost 10 years, demonstrating the durability of response to treatment.56 Definitive prospective data in uveal melanoma is lacking, as these patients were excluded from the initial phase III trials.57,58 One prospective study to determine safety and efficacy of ipilimumab evaluated 82 patients with stage IV uveal melanoma who had failed prior systemic therapy. This study showed an immune-related objective response rate in 5%, immune-related stable disease in 29%, and median overall survival of 6 months.59 When only analyzing the subset of patients with baseline lactate dehydrogenase values <480 U/l, the overall survival increased to 11.6 months. Smaller studies have shown conflicting results with rates of stable disease ranging from 0–44%, and overall survival ranging from 5.2–9.6 months; however, some of these studies had as few as 13 patients and others were retrospective.60–62
The DeCOG trial, a multicenter, single-arm, phase II clinical trial evaluated ipilimumab in patients with metastatic uveal melanoma, is the only published phase II clinical trial to date. The median overall survival was 6.8 months with stable disease in 47%. The 2-year survival rate was only 7%, and they concluded that ipilimumab has limited activity in patients with metastatic uveal melanoma.63 Preliminary results from a phase II trial conducted in Spain showed more promising results. At 5.5 months 7.7% had a partial response and 46.2% had stable disease; however, the overall survival and progression free survival results are still pending.64 Outside of a clinical trial, ipilimumab therapy is a reasonable standard of care treatment option for patients with metastatic uveal melanoma.
PD-1 inhibitors
Pembrolizumab and nivolumab are programmed cell death-1 (PD-1) receptor antibodies approved for the treatment of metastatic melanoma. Compared to ipilimumab, pembrolizumab improved survival, response rates, and had fewer treatment-related adverse events in patients with cutaneous melanoma.65 However, a multicenter retrospective review of patients with metastatic uveal melanoma who had received either a PD-1 or programmed death-ligand 1 (PD-L1) antibody inhibitor, demonstrated that these medications are well tolerated, but the response rates were less promising. The overall response rate was only 3.6% with a median progression free survival of 2.6 months and median overall survival of 7.6 months.66 The poor response to these agents is likely due to the very low rates of PD-1 and PD-L1 expression in uveal melanoma.67 A phase II trial evaluating the safety and efficacy of pembrolizumab in patients with metastatic uveal melanoma is currently recruiting patients (NCT02359851).
Novel immune therapies
Tumor cells evade the immune system by a number of mechanisms, one of which is inducing dysfunction of dendritic cells.68 As a way to enhance immunogenicity of vaccines, dendritic cells can be incorporated as vectors of melanoma-associated antigens (NCT00089219, NCT00313508). A small study of 14 patients with metastatic uveal melanoma evaluated the safety, immunologic response, and overall survival of patients treated with dendritic cell vaccination using gp100 and tyrosinase antigens. The patients exhibited no grade 3 or 4 adverse events. The overall survival was 19.2 months and 29% of patients demonstrated tumor-specific immune response.69
Immune mobilizing monoclonal T-cell receptors against cancer (ImmTACs) enable a patient’s immune system to target tumor cells via a bidirectional biologic drug, IMCgp100. The drug binds the gp100 antigen on melanoma tumor cells while simultaneously binding and activating the patient’s T cells. Preliminary data from a phase I trial demonstrated a favorable safety and response profile in cutaneous and uveal melanoma with a clinical benefit rate of 71%. Of the fourteen evaluable uveal melanoma patients, two patients had a partial response (14%) and eight patients had stable disease (57%).70 Because of these results, IMCgp100 was granted orphan designation for the treatment of uveal melanoma, and phase I trials are ongoing (NCT01211262, NCT02570308).
Targeted therapy
GNAQ and GNA11 mutations affect cell proliferation and apoptosis through activation of the MAPK pathway and downstream effectors including the MEK, PKC, PI3K/AKt/mTOR signaling cascades. Activating mutations of the receptor tyrosine kinase c-kit, an upstream effector, have been implicated in uveal melanoma as well as other cancers. The inhibition of these signaling pathways is a potential rational treatment technique for metastatic uveal melanoma. Currently, these therapies are still considered investigational, and patients can gain access to targeted therapies through clinical trials.
MEK inhibitors
The clinical activity of the MEK inhibitor trametinib (GSK1120212) was evaluated in a phase I trial of patients with solid tumors, which included 16 patients with uveal melanoma, with encouraging results. Of the uveal melanoma patients, eight (50%) had stable disease and four (25%) maintained a durable response of at least sixteen weeks.71
The response rate to the MEK1/2 inhibitor selumetinib was markedly better when compared to chemotherapy in a phase II trial of uveal melanoma patients. In the selumetinib group, 49% of patients demonstrated tumor regression. Tumor regression was not seen in any of the patients in the chemotherapy group. Median progression-free survival was modestly improved with selumetinib compared to chemotherapy (15.9 weeks versus 7.0 weeks, respectively). The improvement in overall survival with selumetinib did not reach statistical significance (9.1 months, 95% confidence interval [CI] 6.1–11.1 for chemotherapy versus 11.8 months, 95% CI 9.8–15.7 for selumetinib); however, the study design allowed patients to receive selumetinib after progression on chemotherapy, which may have confounded the survival data.72 The only phase III trial involving a MEK inhibitor in uveal melanoma compared a MEK inhibitor and dacarbazine to dacarbazine alone, and it did not reach the primary endpoint of an improvement in progression free survival. The data analysis for this trial is still ongoing.73
Conflicting adverse event rates for MEK inhibitors have been reported. A phase I dose-escalation study of an oral MEK inhibitor (TAK-733) demonstrated a tolerable safety profile with only 14% of patients experiencing dose-limiting toxicities.74 However, the phase II trial mentioned above evaluated the oral MEK inhibitor selumetinib in uveal melanoma patients and revealed an adverse event rate of 97% with dose-reduction required in 37%.72 Further studies are necessary to determine the safety and efficacy of these drugs.
Other targets
Sorafenib is a multikinase inhibitor targeting vascular endothelial growth factor (VEGF), platelet derived growth factor receptor, and the RAF family kinases. Sorafenib prolongs overall survival in advanced hepatocellular carcinoma and is currently the only systemic therapy approved for the treatment of metastatic hepatocellular carcinoma.75 Results from the first phase II trial evaluating sorafenib as a single agent in metastatic uveal melanoma were recently published, and the primary endpoint was non-progression rate. Ten of 32 patients (31.2%) showed non-progression at 24 weeks.76 The estimated overall survival was 62.5%, but 41.4% of patients required dose adjustments due to serious adverse events.76 A phase II trial studying sorafenib in combination with chemotherapy has been completed, but the results are not yet available (NCT00329641). A phase II efficacy study of sorafenib versus placebo in chemonaïve patients with metastatic uveal melanoma is ongoing in Germany (NCT01377025).
Another receptor tyrosine kinase inhibitor that is currently undergoing investigation is cabozantinib (XL184), which has multiple targets, though thought to primarily inhibit c-MET and VEGFR2. A phase II randomized discontinuation trial of cabozantinib in patients with nine different types of advanced, recurrent, or metastatic solid tumors been completed, but full results are not yet published (NCT00940225). Results from the uveal melanoma subgroup showed activity of cabozantinib in these patients. Of the 23 patients with uveal melanoma, the median overall survival was 12.6 months and the median progression free survival was 4.8 months.77 A phase II trial comparing cabozantinib-s-malate to temozolomide or dacarbazine was recently halted, and results are not yet available (NCT01835145).
Combination therapies
Inhibiting multiple pathways could lead to a more robust response than is seen with monotherapy. Preclinical studies in vitro and in mouse models have demonstrated that combined inhibition therapy can promote synergy.18–20,78–80 Initial clinical studies have not been confirmatory, however.
Simultaneously inhibiting both MEK and Akt, reduced cell viability in a synergistic manner, in preclinical GNAQ-mutant uveal melanoma mouse models.78 Consequently, this combination of therapies is currently being evaluated in a phase II study.81 Unfortunately, preliminary results from this trial did not show any improvement in progression free survival with combination MEK and Akt inhibitor therapy compared to monotherapy with a MEK inhibitor alone.81 Adverse events were found in 100% of patients, and the combination therapy arm had more grade 3 adverse events. Neither arm had grade 4 or 5 adverse events, but a dose reduction was required in seven of 18 patients using a MEK inhibitor alone and in seven of 21 patients using both MEK and Akt inhibitors in combination. This trial is still recruiting participants (NCT01979523). See Table 2 for selected trials on systemic therapy.
Future directions
Clinical trials to evaluate multiple targeted therapies and therefore the inhibition of multiple pathways simultaneously, are currently underway. Furthermore, combination therapy with diverse groupings of targeted therapy, immune therapy, chemotherapy, and hepatic-directed therapies are under investigation at various stages.
Adoptive cell therapy has shown promising results in cutaneous melanoma, but has not yet been studied extensively in uveal melanoma. This approach infuses tumor specific T cells into a patient with the aim of immune recognition and destruction of tumor cells.82 A phase II trial is currently ongoing to study the safety and efficacy of the infusion of autologous tumor-infiltrating lymphocytes in patients with metastatic uveal melanoma (NCT01814046).
Other novel therapeutics are also being investigated. Glembatumumab vedotin is an antibody-drug conjugate, which combines an antibody directed at the transmembrane glycoprotein NMB (gpNMB) with the microtubule inhibitor monomethyl auristatin (MMAE). Modest clinical activity has been demonstrated in heavily pretreated cutaneous melanoma patients.83 Given the high frequency of gpNMB expression in uveal melanoma, there is a strong preclinical rationale to explore the antitumor activity in this patient population.84 A phase II study evaluating glembatumumab vedotin in patients with uveal melanoma has recently completed accrual (NCT02363283).
The epigenetics of cancer is another therapeutic approach under investigation. Enzymes such as DNA methyltransferases and histone deacetylases are potential targets as they can impact gene expression as well as cell proliferation and differentiation. Preclinical data suggests that histone deacetylase inhibitors could change the aggressive phenotype of uveal melanoma cells in vitro.85 A phase II trial evaluating vorinostat, a histone deacetylase inhibitor, in metastatic uveal melanoma is underway (NCT01587352). The combination of a histone deacetylase inhibitor and pembrolizumab is also currently under investigation in a phase II trial (NCT02697630). See Table 3 for selected ongoing studies.
Conclusion
Metastatic uveal melanoma has a grim prognosis, and currently no standard of care exists to guide management. The molecular profile of uveal melanoma is distinct from cutaneous melanoma, and accordingly the treatments differ. In order to define optimal management, patients diagnosed with advanced uveal melanoma should be offered participation in a clinical trial whenever possible. Advances in knowledge of the genetic/immunologic profile of uveal melanoma have led to the development of novel treatments that are currently under investigation. Future therapies will likely involve an integrated approach, using a combination of treatment modalities.