Hyaluronic acid (HA) is a key component of the stroma that dominates the microenvironment of tumours of several different types of cancer especially metastatic pancreatic ductal adenocarcinoma (PDA).1 It contributes to the quite solid characteristics of these tumours, and is an important factor its poor prognosis and in making the disease difficult to treat.1,2 Pancreatic cancer is the 11th most commonly diagnosed cancer in men and the ninth in women;3 however, it is the third leading cause of cancer death worldwide,3 which is a reflection of its poor prognosis. Pancreatic cancer is almost always fatal, with a 5-year survival rate of around 8%,4 and accounts for more than 90% of all pancreatic tumours. Although overall cancer incidence and death rates are declining, the incidence and death rates for pancreatic cancer are increasing and it is projected to become the second leading cause of cancer-related death in the Western world by 2030.5 This high mortality rate is due to the fact that the disease is often diagnosed at a late stage and it has an extremely aggressive biology.3 Moreover, there is a lack of effective treatment options.
Recently, nab-paclitaxel, an albumin-coated nanoparticle formulation of taxol, in combination with gemcitabine or the combination of 5-fluorouracil (5-FU)/leucovorin (LV), oxaliplatin and irinotecan (FOLFIRINOX) are associated with improved prognosis compared with gemcitabine alone.6,7 However, none of these agents have extended median survival beyond 12 months. Effective treatments to improve this situation represent a serious unmet need.
There is a need for a better understanding of the molecular mechanisms underlying PDA in order to identify novel targets for drug developments. Recent advances in our understanding of the complex interaction between cancer cells and the tumour microenvironment, however, are providing promising new avenues for therapeutic intervention. This review aims to consider the role of HA and other stromal components in the initiation and progression of PDA, as well as exploring the potential of therapeutic targeting of stromal components.
Role of the stroma in pancreatic cancer
Stromal cells are found in all organs of the body and support the parenchymal cells of that organ. The interaction between stromal cells and tumour cells plays a major role in tumour growth and progression. A defining characteristic of PDA is its high stromal content: up to 90% of the tumour volume. This comprises a complex set of cells including cancer associated fibroblasts (CAF) and pancreatic stellate cells (PSC), immune cells, vascular cells and inflammatory cells surrounded by an abundant quantity of extracellular matrix (ECM), as well as soluble proteins such as cytokines and growth factors.8–10 A complex interplay occurs between the cancer cells and the various components of the tumour microenvironment and recent studies have focussed on defining how the stromal components contribute to tumourigenesis.
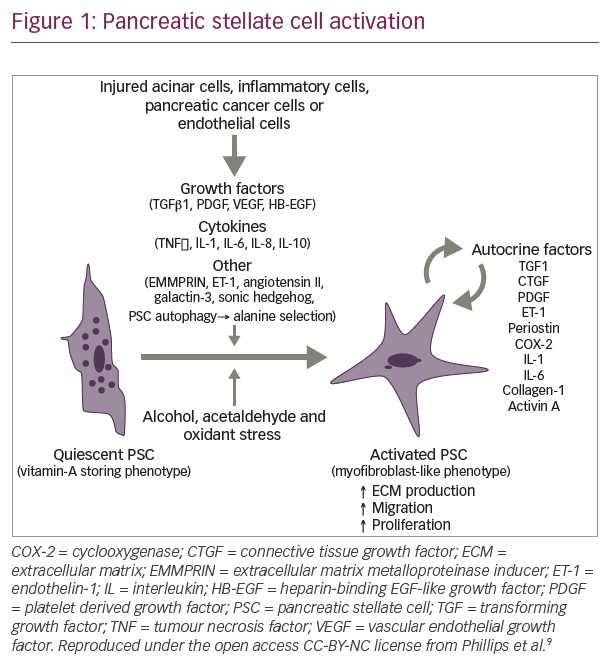
Among the most important cellular components of the stroma are PSCs. In their quiescent state PSCs contain lipid droplets and act as a reservoir for vitamin A. However, in PDA they transform into an active myofibroblast-like phenotype, which involves changes to their morphology and proliferation rate, part of a process known as desmoplasia (Figure 1).9,11 Activation of PSCs involves a number of intracellular signals including mitogen-activated protein kinases, phosphatidylinositol kinase, protein kinase C, peroxisome proliferator activated receptor gamma, the Janus kinase activator transcription (JAK/STAT) pathway and various transcription factors.9,12 In addition, desmoplasia involves increased expression of transforming growth factor β, basic fibroblast growth factor connective tissue growth factor and interleukin-1β, leading to increased deposition of ECM proteins.11 During disease pathogenesis, PSCs remain in the activated state through exposure to cytokines and growth factors produced by acinar cells, inflammatory cells, platelets, endothelial cells, cancer cells and PSCs themselves.13 In turn, PSCs provide important factors to the tumour cells which they need in an environment lacking important nutrients. Recently, it was demonstrated that PSCs support tumour metabolism through autophagic alanine secretion.14
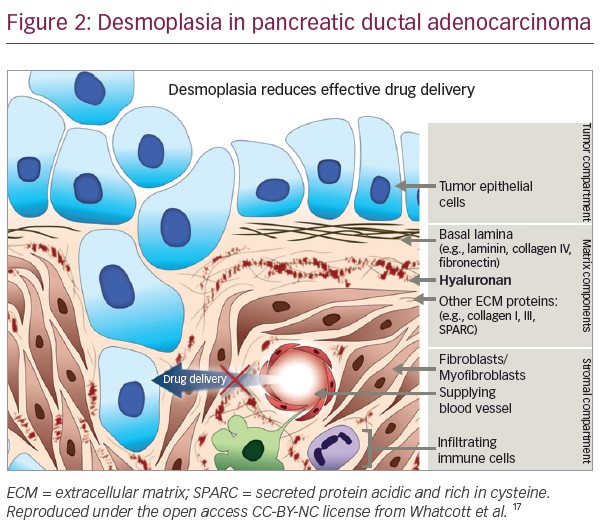
Desmoplasia results in the production of dense, fibrous connective tissue in the ECM, which reduces the elasticity of tumour tissue, and increases tumour interstitial fluid pressure (IFP), distorting the normal architecture of pancreatic tissue and inducing an abnormal configuration of blood and lymphatic vessels.11,15 This increased IFP induces vascular compression and collapse in the immediate vicinity of the tumour, as well as impeding access of even small molecule therapeutic agents and harbouring infiltrative macrophages and inflammatory cells that can suppress cancer-directed immune mechanisms.11,16,17 In addition, stromal cells promote the migration, growth and invasion of tumour cells.9
Targeting the stroma – friend or foe?
The role of the stroma in inhibiting drug delivery in PDA has led to its investigation as a therapeutic target. However, studies investigating the impact of targeting the stroma in animal models found that depletion of myofibroblasts led to invasive, undifferentiated tumours with enhanced hypoxia, epithelial-to-mesenchymal transition and cancer stem cells, as well as diminished survival.18,19 In addition, lower levels of myofibroblasts in the tumours of patients with PDA have been associated with lower survival.18 In another animal study, the suppression of sonic hedgehog (Shh), a soluble ligand overexpressed by neoplastic cells in PDA that is associated with desmoplasia, reduced the stromal content of tumours but increased the proliferative ability of the tumours.19 These two studies suggest that some elements of the stroma are, in fact, protective and targeting them can lead to a more aggressive form of PDA20 that may then require a more aggressive treatment. Given the complexity of the stroma and associated immune cells, there is a need to identify deleterious and beneficial aspects of stroma biology in PDA.
The role of hyaluronic acid in pancreatic cancer
Among the stromal components, the role of HA in cancer initiation and progression has been extensively studied. HA is an extracellular glycosaminoglycan comprising repeating disaccharide units of N-acetylglucosamine and D-glucuronic acid. It is produced by HA synthases, degraded by hyaluronidase, is naturally found in the extracellular spaces of most tissues and is highly hydrophilic, binding water to create a viscous gel.21 Its function was believed to be to maintain the volume and rigidity of connective tissue;17,21 however, the discovery of HA-binding proteins and surface receptors suggest further roles in biological processes.22 Matrix proteoglycans and glycoproteins cross-link hydrophilic HA to the ECM. This cross-linked matrix then sequesters growth factors and cytokines in the tumour microenvironment.1
The discovery of HA synthase (HAS) genes, which encode the key enzymes in HA biosynthesis, has enhanced our understanding of the mechanism underlying altered HA production in cancer. In vitro studies have shown that increased cellular HA production in canine kidney and human mammary epithelial cells increased anchorage-independent growth and invasiveness, induced gelatinase production and stimulated the phosphoinositide 3-kinase/protein kinase B (Akt) pathway activity. In addition, cells infected with HA synthase-2 adenovirus acquired mesenchymal characteristics.23 In another study, HA induced the differentiation of epitheloid mesothelioma cells to a fibroblastic phenotype. This study also found that increased HA synthesis promoted greater cell proliferation, migration and anchorage-independent growth.24 Studies using HAS transgenic mice have shown that overproduction of HA in mammary tumours accelerates tumour growth through the recruitment of stromal cells and vasculature.25
In normal physiological conditions, the amount of HA is controlled by a balance between synthesis and degradation. However, in many types of malignant tumours, HA is often overexpressed or highly concentrated in tumour cells, particularly in the stromal compartments, where it stabilises the tumour microenvironment.21,25 Expression of HA has been reported in 25−30% of human tumours and in up to 87% of PDAs.1 It has also been detected in higher levels in the peripheral invasive area of tumours compared with the central non-invasive area, suggesting it plays a role in invasion.17,26 The HA receptor CD44 is overexpressed in tumour cells and is believed to play an important role in angiogenesis and the invasive process.27,28 Animal studies have found that induction of HA overproduction accelerates angiogenesis through stromal cell recruitment.29 Furthermore, in vitro studies have found that HA stimulates PDA cell migration.30
The cellular origin of HA in tumours is varied and includes stromal fibroblasts as well as tumour cells, with the interaction between the two appearing to play a key role in HA accumulation.26,31 HA can be produced by pancreatic cancer cells via an epigenetically regulated mechanism but also by PSCs. The mechanisms regulating synthesis and degradation of HA in PDA are complex and a matter of intense research.32–34 Accumulation of HA is associated with rapid tumour progression and is an independent negative predictor of survival in a number of solid tumour types.35–40 This principle was shown in a study that employed immunohistochemistry to analyse the expression of HA and its regulators. HA was strongly expressed in 78% of primary PDA tissues, with staining being detected both in tumour and stromal components. Such strong HA expression was significantly associated with shorter survival times after surgery.41 Another study assessed desmoplasia in metastatic lesions of PDA and compared it with that of primary tumours. HA was predominantly associated with the desmoplastic stroma rather than with tumour cells and was found in high levels in both metastatic and primary tumours, with no significant difference between the two. A significant negative correlation was found between patient survival and ECM deposition in primary tumours.42
In addition to its role in tumour progression, HA has also been identified as the primary determinant of the barrier to the access of therapeutic agents (Figures 2 and 3).11,16,17 In addition to contributing to the increased tumour IFP and vascular compression described in the previous section, the matrix produced by HA-high cells also inhibited immune cell access to tumour cells in vitro.43–45 These findings suggest that targeting HA represents a novel therapeutic approach to combat the chemoresistance of PDA.
Targeting hyaluronic acid in pancreatic cancer
Three therapeutic approaches to targeting HA have been proposed: (1) inhibiting HA synthesis (4-methylumbelliferone is the most studied agent but development is still at the preclinical stage),46 (2) blocking HA signalling using HA oligosaccharides (again, studies are at the preclinical stage)21 and (3) depleting stromal HA in PDA.21 The latter appears to be a particularly attractive strategy – and is the most studied – but early studies using bovine hyaluronidase were limited by adverse reactions and poor efficacy in vivo.17,47 More recently, a pegylated recombinant human hyaluronidase (pegvorhyaluronidase alfa), which increased bioavailability and penetration into tissues, has been developed.48–50
In a number of preclinical studies, pegvorhyaluronidase alfa depleted stromal HA, reduced interstitial fluid pressure, caused re-expansion of the tumour vasculature, reduced tumour hypoxia and improved macromolecular permeability, resulting in inhibition of tumour growth.48–50 Pegvorhyaluronidase alfa has also been found to reduce hypoxia-related protein expression and inhibit the translocation of E-cadherin and β-catenin to the plasma membrane.48 In a study using murine models, pegvorhyaluronidase alfa administration improved vessel patency and increased intra-tumoural delivery of doxorubicin and gemcitabine. Furthermore, combination therapy with gemcitabine and pegvorhyaluronidase alfa led to improved survival relative to gemcitabine alone (28.5 versus 15.0 days, p=0.002).51 In combination with gemcitabine, pegvorhyaluronidase alfa remodelled the tumour microenvironment and yielded objective tumour responses, resulting in a twofold increase in overall survival.16 Treatment with pegvorhyaluronidase alfa has also been shown to increase antibody-dependent cell-mediated cytotoxicity43 and to enhance anti-PD-1 and anti-PD-L-1 efficacy (assessed as tumour growth) in animal models.44 Moreover, in a murine model, the combination of pegvorhyaluronidase alfa and recombinant live-attenuated double-deleted strains of Listeria monocytogenes (LADD-AH1) resulted in enhanced anti-tumour activity compared with either pegvorhyaluronidase alfa or LADD-AH1 alone.45 The proprietary, live-attenuated, double-deleted strain of the Gram-positive bacterium Listeria monocytogenes (Lm) encodes multiple, but undisclosed, tumour-associated antigens (TAAs), with potential immunostimulatory and antineoplastic activities. Two genes contributing to the virulence of Lm have been removed to minimise the risk of infection.52
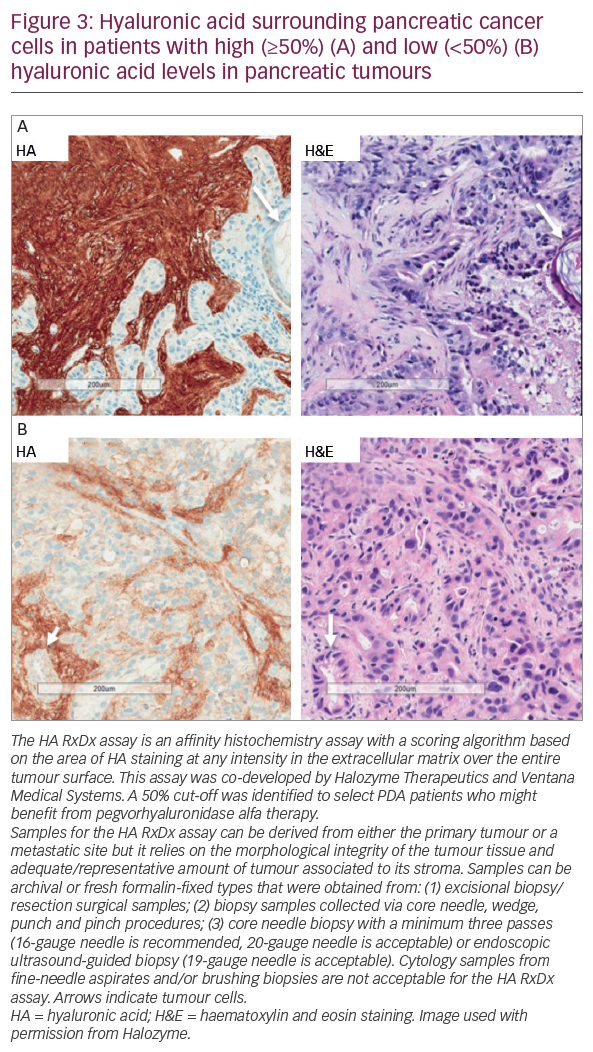
In a phase Ib study, pegvorhyaluronidase alfa (3 μg/kg) in combination with gemcitabine was well tolerated and demonstrated therapeutic benefit in 28 patients with untreated stage IV PDA.53 A subsequent phase II study (the HALO-109-202 study, NCT01839487, n=279) comprised two stages.54 In stage one, patients (n=279) were randomised 1:1 to pegvorhyaluronidase alfa plus nab-paclitaxel and gemcitabine (PAG) or nab-paclitaxel and gemcitabine (AG) chemotherapy alone. This study was also novel in investigating the utility of HA as a potential biomarker to identify patients most likely to respond to pegvorhyaluronidase alfa treatment using the Ventana diagnostic assay (Figure 3). During this stage, the incidence of thromboembolic events (TEs) in the pegvorhyaluronidase alfa arm led to treatment discontinuation in around 40% of patients, leading to an imbalance and temporary halt of the trial in 2014. Many solid tumours including PDA are associated with a hypercoagulable state in which chemotherapies and pegvorhyaluronidase alfa can increase the risk of TEs. This is especially true in patients who are already at risk of such events. The underlying pathology of PDA and the effects of treatment are complex with multiple confounders and as yet no mechanism for this increased TE risk has been proposed. The study protocol was therefore revised to exclude patients at high thromboembolic risk and to include prophylactic enoxaparin (1 mg/kg/day). Stage two of the study followed the revised protocol and randomised patients 2:1 to PAG or AG. The primary endpoints were progression-free survival (PFS) and the incidence of TEs.54
Interim data of 256 patients with HA showed that PFS was statistically higher in the PAG versus AG group (6.0 months versus 5.3 months, hazard ratio [HR]: 0.73, 95% confidence interval [CI]: 0.53–1.00; p=0.048). This difference was considerably more pronounced in the HA-high patient subgroup (n=84, 9.2 months versus 5.2 months; HR: 0.51; 95% CI: 0.26–1.00; p=0.048). The overall response rate (ORR) in patients with high HA tumours was 46% (PAG) versus 34% (AG). In addition, an exploratory analysis of the data suggested that in patients with high-HA tumours, identified using a tissue-based immunohistochemistry assay, the overall survival (OS) was 11.5 months in the PAG group versus 8.5 months in the AG group.54
Since HA is present in all parts of the body (including cartilage) and considering its safety and toxicity profile, the use of hyaluronidase involves inherent risks of side effects. In early phase I studies, dose-limiting toxicities were identified including PDA, muscle spasms, arthralgia and myalgia. A dosing regimen of ≤3 μg/kg administered twice weekly was tolerated with intermittent grade 1–2 musculoskeletal events.55 In the HALO-109-202 study the incidence of TEs were similar in both groups (14% in PAG versus 10% in AG) following enoxaparin initiation. Treatment-related adverse events (AEs) included peripheral oedema (63% in PAG versus 25% in AG), muscle spasms (56% versus 3%), neutropenia (34% versus 19%) and myalgia (26% versus 7%)54.
The US Food and Drug Administration has granted orphan drug designation to pegvorhyaluronidase alfa for the treatment of PDA.56
Future directions
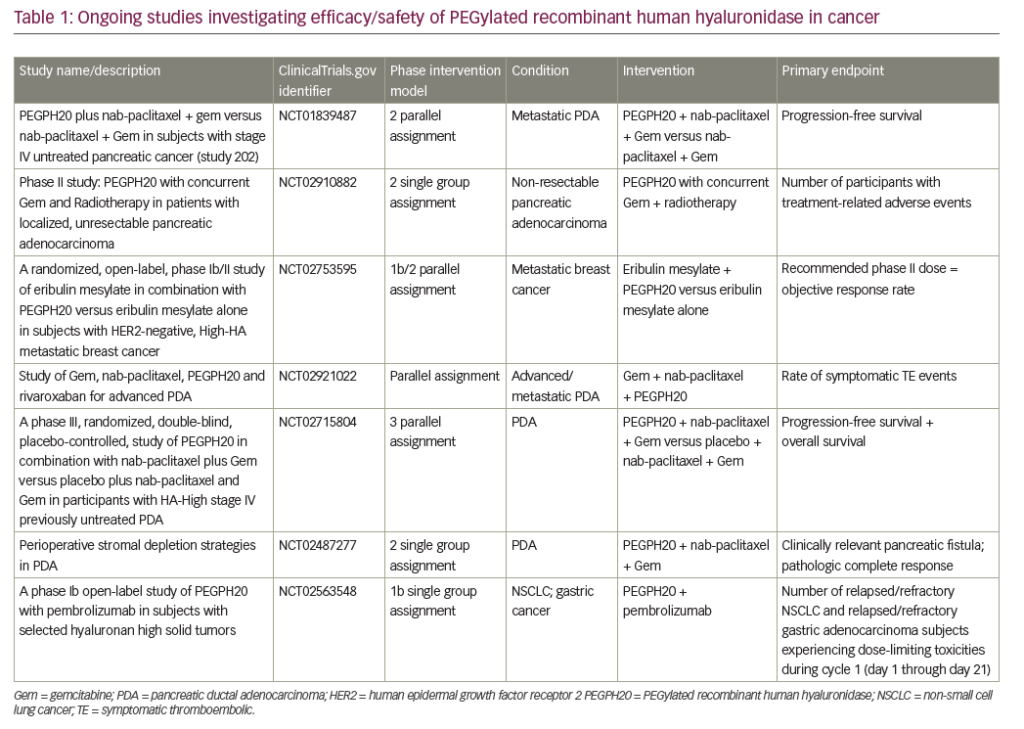
A number of ongoing clinical trials are investigating the use of pegvorhyaluronidase alfa in PDA. The international, double-blind, phase III HALO-109-301 trial using core biopsies is currently recruiting patients with HA-high PDA and includes overall survival as a primary endpoint in addition to PFS (NCT02715804). A pilot study investigating the combination of gemcitabine, nab-paclitaxel and pegvorhyaluronidase alfa, along with the oral anticoagulant rivaroxaban in patients with advanced PDA, is currently recruiting (NCT02921022). Recently, however, a phase I/II trial investigating the combination of mFOLFIRINOX (folinic acid, fluorouracil, irinotecan hydrochloride, oxaplatin) chemotherapy and pegvorhyaluronidase alfa (NCT01959139) in a non-biomarker selected population was stopped for futility at interim analysis. Further analysis of the initial data set is underway, including a planned analysis by HA levels, following completion of retrospective determination of tumour HA levels.57
A phase Ib open-label study is evaluating the combination of pegvorhyaluronidase alfa and pembrolizumab in patients with HA-high tumours. These tumours are relapsed/refractory stage IIb/IV non-small cell lung cancer (NSCLC) or relapsed/refractory metastatic gastric adenocarcinoma that failed ≥1 previous chemotherapy regimen. HA levels were determined using a companion diagnostic assay developed in collaboration with Ventana Medical Systems, Inc. (Oro Valley, Arizona, US) (Figure 3).58
The co-developed Ventana HA companion diagnostic has shown reproducibility for selection of patients with HA-high tumours.59 However, it is only one of a number of potentially predictive biomarkers for PDA under prospective evaluation. This biomarker will allow the identification of patients most likely to benefit from pegvorhyaluronidase alfa. The Precision Promise consortium aims to change the PDA treatment paradigm through the molecular profiling of tumours. Participating patients will enrol at a local Clinical Trial Consortium site. Each patient will undergo molecular profiling to determine which Precision Promise sub-study will best match their individual needs.60
The potential of HA-targeted therapies also extends beyond PDA. Pegvorhyaluronidase alfa in combination with eribulin for the treatment of breast cancer is being evaluated in a phase I clinical trial (NCT02753595). In addition, preclinical studies using pegvorhyaluronidase alfa in combination with atezolizumab in the treatment of gall bladder tumour have been completed and a phase I clinical trial is planned.
Other potential strategies targeting HA include inhibiting HA degradation, since oligosaccharides of HA, produced through degradation by hyaluronidase, have been associated with lymphatic vessel invasion by tumour cells and the formation of lymph node metastasis.61 Although further preclinical and clinical studies are needed, controlling the accumulation of HA by modulating both the production and degradation process represent a promising therapeutic strategy.
Summary and concluding remarks
By creating barriers that separate tumour cells from circulating immune cells and therapeutic agents, the stromal compartment of PDA is largely responsible for the therapeutic resistance and poor prognosis that characterise the disease. Targeting stromal components in PDA and other solid tumours has the potential to improve the delivery and efficacy of current cytotoxic drugs, although some elements of the stroma have been found to restrain tumour growth and targeting them can result in a more aggressive form of PDA, leading to increased metastases. Targeting HA may, therefore, be both deleterious and beneficial to the progression of PDA. However, preclinical evidence suggests that HA represents a useful therapeutic target as a result of its documented role in tumour progression. In addition, a growing body of clinical evidence demonstrates the benefits of the enzymatic agent pegvorhyaluronidase alfa in depleting stromal HA and increasing the efficacy of both chemotherapy and immunotherapy, improving the prognosis of this fatal disease. The addition of pegvorhyaluronidase alfa to nab-paclitaxel and gemcitabine resulted in significantly longer PFS in patients with advanced PDA compared with nab-paclitaxel and gemcitabine alone in a phase II trial. Currently, a phase III trial in patients with advanced PDA is examining whether the prolonged PFS translates into improved overall survival (NCT02539537). Furthermore, HA is important in some other cancer types as well as PDA and the potential of pegvorhyaluronidase alfa in some of those diseases is being investigated. The identification of a subgroup of patients who express high levels of tumour HA, and are therefore more likely to benefit from this treatment, has dramatically improved outcomes in studies investigating pegvorhyaluronidase alfa. In addition to PDA, HA-targeting therapies have the potential to extend survival in a large number of patients with cancer.