In 2015, more than 430,000 people in the US were living with lung cancer and there were around 221,200 new cases of lung cancer. Deaths from lung cancer are estimated to be in the region of 158,040.1 Lung cancer is the most frequent cause of cancer deaths in men globally and, in women, lung cancer has surpassed breast cancer as the leading cause of cancer death in developed countries.2 Data from the National Lung Cancer Audit (LUCADA) for England in 2011 show that the majority of lung cancers (87%) are classified as non-small cell lung cancer (NSCLC).3 Forty per cent of all NSCLC cases present with Stage III cancer and many of them will be considered inoperable.4
Patients with stage I to IIIa NSCLC are usually treated curatively using surgery, chemotherapy, radiation or a combined modality approach. Patients with advanced disease are generally treated with systematic chemotherapy, although response and survival rates are suboptimal; survival rates for patients diagnosed with stage IIIB and IV NSCLC are just 5% and 1%, respectively.5,6 Further, only a small proportion of patients benefit from later-line therapies.7
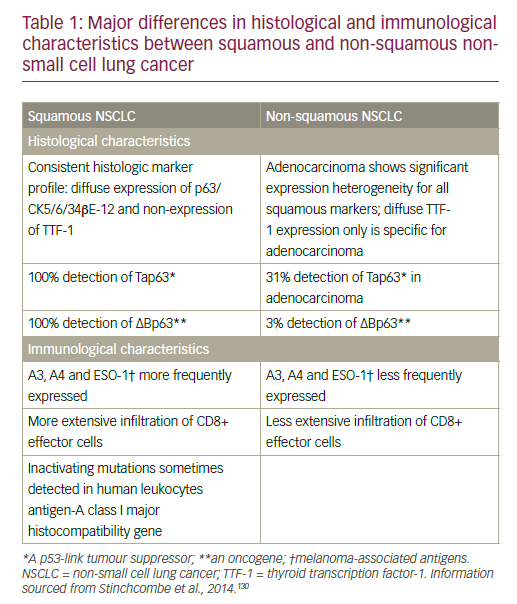
NSCLC comprises different histological types that are divided into two main groups that inform treatment decision-making: non-squamous carcinoma (including adenocarcinoma, large-cell carcinoma, and other cell types) and squamous cell carcinoma.8 Differences in histological and immunological characteristics of squamous and non-squamous NSCLC are summarised in Table 1.
Non-squamous cell NSCLC has benefited from the development of targeted therapies for specific molecular subsets with epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements. Squamous cancers account for between 20% and 30% of NSCLC cases and are still treated with cytotoxic chemotherapy alone.9–11 Adenocarcinoma, the most common subtype of NSCLC, accounts for approximately 40% of lung cancers in the US, with rising prevalence also seen in Europe.12,13 Squamous cell carcinoma is closely related to smoking and has a distinct and more complex genetic signature compared with non-squamous tumours.9 In a National Institutes of Health/AARP cohort of 186,057 women and 266,074 men aged between 50 and 71 years, who were followed for 11 years, relative risks for current smoking and incidence of smoking-related cancers were similar in men and women, probably reflecting converging patterns in smoking.14 Asthma, chronic obstructive pulmonary disease (COPD) and tuberculosis (TB) were associated with an increased risk of all major subtypes of lung cancer in a Taiwanese population-based study.15 Women with TB carried the highest risk in this analysis.
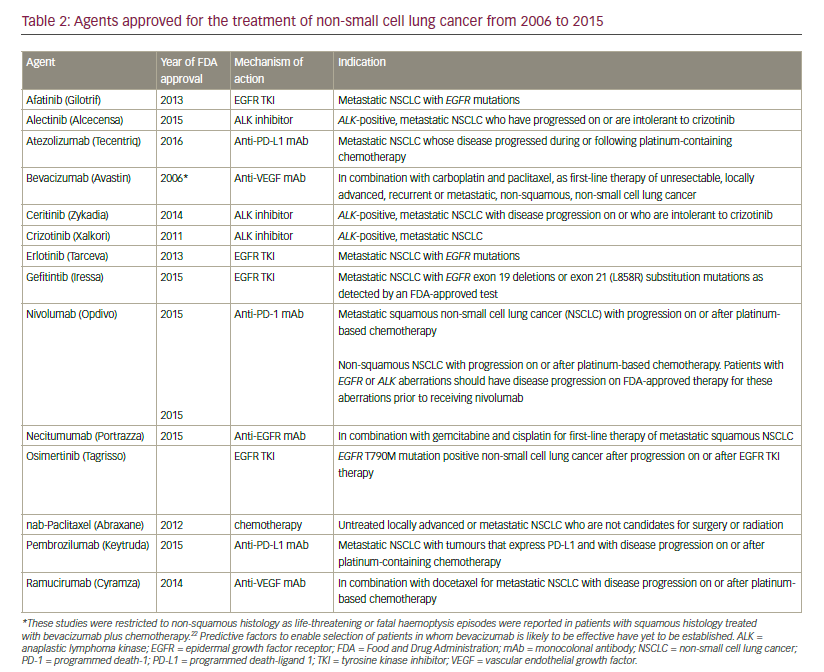
Advances in the development of targeted therapies and immunotherapy has led to approval by the US Food and Drug Administration (FDA) for 12 agents in the last 10 years (see Table 2). This review aims to review these breakthroughs in the management of NSCLC.
Angiogenesis as a target
A balance between pro-angiogenic and anti-angiogenic factors regulates angiogenesis in both physiologic and pathologic conditions.16 In many cancers, including NSCLC, proangiogenic pathways have become established as important and effective therapeutic targets because
they are essential for tumour growth, progression and metastasis.17 Antiangiogenics improve tumour oxygenation, thereby improving the therapeutic efficacy of irradiation in models.4 Angiogenesis is regulated by complex signalling pathways with multiple cytokines and growth factors, the most important of which are vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF).18,19 Three categories of antiangiogenic therapeutic agents have been identified:20
• direct agents targeting endothelial cells and their functions (e.g. proliferation, migration and the formation of new vessels);
• indirect antiangiogenic drugs that target cancer cells by interfering with the production of angiogenic factors or extracellular processes; and
• mixed drugs that are aimed at both endothelial and tumour cells.
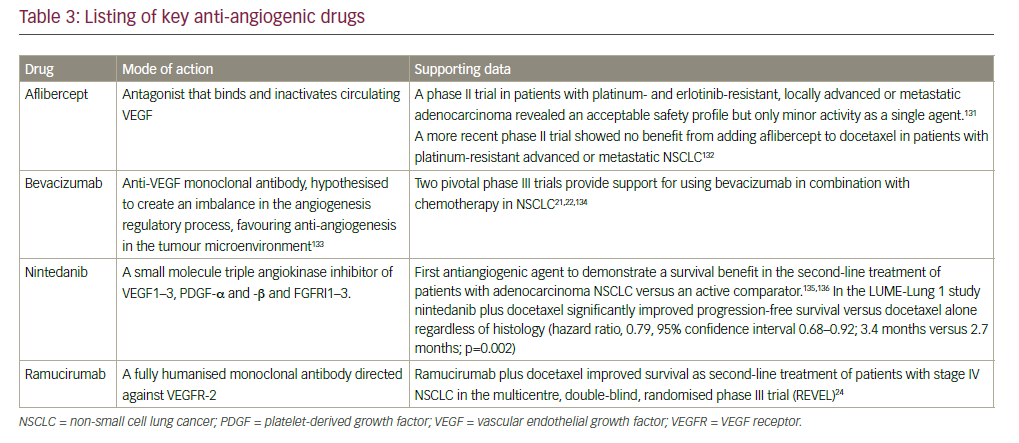
The major characteristics of a selection of antiangiogenic compounds are summarised in Table 3.
In 2006, the FDA granted approval for the use of bevacizumab, a monoclonal antibody targeting VEGF, in combination with carboplatin and paclitaxel for the initial systemic treatment of patients with unresectable, locally advanced, recurrent or metastatic, non-squamous NSCLC, following data from the Eastern Cooperative Oncology Group ECOG 4599 study.21 However, the AVAIL study did not show a significant benefit for bevacizumab.22 Bevacizumab is contraindicated in patients with squamous histology tumours and also in central tumours irrespective of histology, owing to a prohibitively high rate of severe pulmonary haemorrhage associated with bevacizumab treatment.23 Other approved anti-angiogenesis monoclonal antibodies include the anti-VEGFR2 agent ramucirumab whose approval was based on the randomised, doubleblind, phase III Study of Docetaxel and Ramucirumab Versus Docetaxel and Placebo in the Treatment of Stage IV Non-Small Cell Lung Cancer Following Disease Progression After One Prior Platinum-Based Therapy (REVEL) trial, in which ramucirumab improved overall survival (OS) in combination with docetaxel compared with docetaxel alone in the secondline setting of NSCLC24 and nintedanib, which targets PGDF receptor (PGDFR), fibroblast growth factor receptor (FGFR) and VEGF receptor (VEGFR), and showed improved OS compared with docetaxel alone in the second-line setting of NSCLC, only in patients with adenocarcinoma in the BIBF 1120 Plus Docetaxel as Compared to Placebo Plus Docetaxel in 2nd Line Non Small Cell Lung Cancer (LUME-lung 1) trial.25
Targeting molecular drivers of carcinogenesis
In the last decade, an increased understanding of the molecular drivers of carcinogenesis has led to a paradigm shift in the management of NSCLC. The discovery of driver mutations in oncogenes, such as the EGFR, ALK, Kirsten rat sarcoma viral oncogene (KRAS), ROS1 protooncogene receptor tyrosine kinase (ROS1) and V-raf murine sarcoma viral oncogene homolog B1 (BRAF), has resulted in the clinical development of numerous targeted therapies.
Epidermal growth factor receptor
EGFR, which belongs to the ErbB family of receptor tyrosine kinases (RTK), is over expressed in between 40% and 80% of NSCLC patients and has been shown to be associated with poor prognosis.26,27 Its activation has been implicated in cellular proliferation, apoptosis inhibition, angiogenesis, metastases and chemoradio-resistance.28 It is supposed that the blockade of EGFR activation by tyrosine kinase inhibitors (TKIs), monoclonal antibodies, ligand-linked toxins and antisense approaches may ultimately lead to inhibition of cancer cell proliferation.29 The incidence of EGFR mutation varies with ethnicity, with up to 50% of tumours being driven by activating EGFR mutations in Asian populations compared with 10% to 15% in Caucasians.30,31 Detection of EGFR mutations has involved tissue biopsy but less invasive methods are becoming available, including the use of circulating tumour DNA.
The use of EGFR TKIs as first-line therapy for patients with advanced EGFR mutation-positive NSCLC began with gefinitib,32–35 followed by erlotinib.36,37 Both are reversible competitive inhibitors of adenosine triphosphate (ATP) for the tyrosine kinase domain of EGFR resulting in blockade of downstream pathways. Second-generation EGFR TKIs include afatinib,38,39 which is approved for first-line treatment of EGFR-mutated tumours and dacomitinib; the latter showed promising efficacy in a phase II trial41 but failed to improve outcomes in two phase III trials in unselected populations.
Until recently, there were no head-to-head trials of EGFR TKIs, but the results of two trials have recently been presented. A study comparing gefinitib and afatinib showed that afatinib significantly improved efficacy versus gefitinib across a number of outcome measures, including progression-free survival, time-to-treatment failure and objective response rate.41 The primary analysis of OS data showed no advantage for afatinib (ESMO 2016). In a post-hoc analysis of two trials of afatinib versus chemotherapy in EGFR-positives, an OS benefit for afatinib firstline was detected in patients with Exon 19-deletions, but not for the Exon 21 point mutation L858R.42 This is the first proof that the sequence of therapy (i.e. afatinib first) matters. This could not be shown for either erlotinib or gefitinib.43 In the phase III LUX-Lung 8 trial, afatinib significantly improved OS in ‘wild type’ EGFR patients compared to erlotinib, reducing the risk of death by 19% in patients with advanced squamous cell NSCLC, who were previously treated with first-line chemotherapy.44
Almost all patients with initial response to EGFR TKIs eventually relapse due to acquired resistance.45 Around half of patients develop secondary resistance within 9 to 12 months of starting an EGFR TKI.46 Around
half of resistance to EGFR TKIs has been attributed to a recurrent missense mutation: T790M within the EGFR kinase domain,47,48 the rest are accounted for by EGFR point mutations, EGFR amplification, bypass tracks and unknown mechanisms in 15–20%.49 Third-generation EFGR inhibitors in clinical development target the T790M mutation; these include rociletinib (CO-1686)50 osimertinib (AZD9291)51,52 and olmutinib (HM61713).53 Osimertinib received FDA and EMA approval in this setting in 2015. In addition, osimertinib has demonstrated high efficacy (ORR 75%, 72% of PFS at 12 months) in preliminary data in the first-line setting.54,55 Other mechanisms of resistance can currently only be treated with chemotherapy: these include the activation of alternative signalling pathways (Met, hepatocyte growth factor [HGF], AXL, Hedgehog (Hh), insulin-like growth factor 1 receptor [IGF-1R]), alterations to downstream pathways (AKT mutations, loss of PTEN), impairment of the EGFR-TKIs-mediated apoptosis pathway and histological transformation.56
Anti-EGFR monoclonal antibodies bind EGFR on the surface of tumour cells and block the binding of EGF. Monoclonal antibodies may also act via immunological mechanisms, for example, antibody-dependent cellular cytotoxicity.57
Cetuximab is a monoclonal antibody targeted at the EGFR signalling pathway. Two randomised, open-label phase III trials compared chemotherapy and cetuximab with chemotherapy alone in patients with advanced NSCLC.58,59 Improved OS for cetuximab added to chemotherapy was shown in the Cetuximab plus chemotherapy in patients with advanced non-small cell lung cancer (FLEX) trial; in contrast, the BMS099 trial failed to demonstrate an improvement in progression free survival. A meta-analysis of individual patient data concluded that the combination of cetuximab plus chemotherapy significantly improved clinical outcomes including OS, and had an acceptable safety profile, however, due to the small benefit, approval was not pursued by the company.60
In 2015, another monoclonal antibody, necitumumab, was approved for the first-line treatment of metastatic squamous NSCLC in combination with gemcitabine and cisplatin. In Europe, approval was given for necitumumab in combination with gemcitabine and cisplatin chemotherapy is indicated for the treatment of adult patients with locally advanced or metastatic EGFR expressing squamous non-small cell lung cancer who had not received prior chemotherapy for this condition. Approval was based on data from the First-line Treatment of Participants With Stage IV Squamous Non-Small Cell Lung Cancer With Necitumumab and Gemcitabine-Cisplatin (SQUIRE) trial, studied necitumumab in combination with cisplatin and gemcitabine in patients with squamous cell NSCLC (n=1,093).61 The primary endpoint of OS was improved by the addition of necitumumab to chemotherapy. The hazard ratio (HR) was 0.84. Median survival times were 11.5 and 9.9 months for the chemotherapy and necitumumab arm and versus chemotherapy alone, respectively, one-year survival rates were 47.7% versus 42.8% and two-year survival rates were 19.9% and 16.5%. Progression-free survival was also improved (HR 0.85; p=0.02). The survival benefit was more pronounced in the German SQUIRE subpopulation with EGFR-expressing tumours than in the overall (intention-to-treat) population (HR 0.59, p=0.026).62 In another subpopulation of patients with EGFR protein expression, OS for EGFR >0 patients was significantly longer in the necitumumab plus gemcitabine-cisplatin group than in the gemcitabine-cisplatin group (HR 0.79 [95% confidence interval (CI) 0.69, 0.92; p=0.002]; median 11.7 months [95% CI 10.7, 12.9] versus 10.0 months [8.9, 11.4]).63 The First-line Treatment of Patients With Stage IV Nonsquamous Non-Small Cell Lung Cancer With Necitumumab and Pemetrexed-Cisplatin (INSPIRE) trial investigated cisplatin and pemetrexed with and without necitumumab in patients with advanced non-squamous cell NSCLC.64 The trial was prematurely closed due to an increased number of fatal thromboembolic events in patients in whom necitumumab had been added to cisplatin. No statistical different in OS and progression-free survival were apparent between the two study arms.
Anaplastic lymphoma kinase
In 2007, a rearrangement in the ALK gene was reported in 6.7% (five out of 75) of NSCLC patients.65 Inversion in the short arm of chromosome 2 fuses the N-terminal domain of echinoderm microtubule-associated protein like 4 (EML4) to the intracellular kinase domain of ALK. This results in a fusion gene that causes constitutive activation of the tyrosine kinase, which is implicated in uncontrolled cell growth and proliferation. Patients with EML4-ALK positive NSCLC tend to be younger and are more likely to have ever smoked than those who do not have the ALK rearrangement. The prevalence of ALK gene rearrangement is about 4–5% of all NSCLC patients and appears to be similar between patients of Caucasian and Asian ethnicity.66 The ALK inhibitor crizotinib was approved by the FDA in 2011 and has become the standard of care in ALK-positive NSCLC,67,68 but resistance inevitably develops, leading to the development of second-line ALK inhibitors ceritinib69 and alectinib,70,71 both of which have been approved by the FDA and are now being investigated in the first-line setting. Recently presented data showed that patients treated with first-line ceritinib had a 45% reduction in the risk for progression of advanced ALK-positive NSCLC compared with chemotherapy.72 Data from the phase III J-ALEX study shows that alectinib demonstrated significantly prolonged PFS (not reached versus 10.2 months) compared with crizotinib and was well tolerated.73 Although ALK inhibitors are generally well tolerated, they are associated with a wide range of treatment-emergent adverse events (AEs), including gastrointestinal AEs and hepatotoxicity, but most are manageable and reversible.74
Several other agents are in clinical development. The dual ALK/EGFR inhibitor brigatinib showed promising activity in ALK-positive NSCLC patients with brain metastasis following crizotinib.75 Other agents include ASP3026,76 X-396,77 TSR-011 and the dual ALK/ROS 1 inhibitor lorlatinib (PF-06463922).78
ROS1
Another molecular subgroup, around 1% of NSCLC patients,72 have chromosomal rearrangements of the gene encoding ROS1 protooncogene receptor tyrosine kinase (ROS1). Following evidence suggesting that that ROS1 is another therapeutic target of the ALK inhibitor crizotinib,80 crizotinib was approved in this patient subpopulation after demonstrating an ORR of 72% in patients with advanced ROS1– rearranged NSCLC.81
BRAF
Mutations of the BRAF gene have been identified in 1% to 2% of patients with NSCLC,82 leading to the investigation of the BRAF inhibitor dabrafenib, which has demonstrated an overall response rate (ORR) of 63% in combination with the MEK inhibitor trametinib.83 An ORR of 42% was also reported in a phase II study of vemurafenib.84
KRAS
Activating mutations in KRAS are found in about 30% of adenocarcinoma and 4% of squamous cell carcinomas.85 However, development of effective KRAS inhibitors has proved challenging. Selumetinib, a MEK1/ MEK2 inhibitor, showed promising efficacy in combination with docetaxel in a phase II trial,86 however, the consecutive phase III trial failed to meet its primary endpoint.87
Other molecular driver targets
Other molecular targets are currently under investigation. These include ROS1 fusions, RET fusions, neurotrophic tyrosine kinase receptor type 1 (NTFK1) fusions, Met gene amplification, FGFR1 gene amplification, HER2 mutations.88 Amplification of mesenchymal-epithelial transition (MET) factor is found in about 5% of lung adenocarcinoma. Promising data were obtained from two phase II trials, one investigating dual EGFR and MET inhibition, with erlotinib and tivantinib,89 the other evaluating the monoclonal antibody onartuzumab.90 As a result, phase III trials were initiated but both were terminated early due to lack of efficacy. Other oncogenic alterations with potential as therapeutic targets include include RET fusions, neurotrophic tyrosine kinase receptor type 1 (NTFK1) fusions, FGFR1 gene amplification and HER2 mutations.88
Despite demonstrating impressive efficacy, targeted therapies have limitations. In many cases, known mutations are not present: squamous NSCLC rarely have EGFR and ALK mutations.91 There is a need to identify driver mutations; the Lung Cancer Mutation Consortium was formed to enable collaborative multi-institutional analyses of ten potential oncogenic driver mutations. Among 1,007 patients on whom mutation analysis were performed, EGFR, KRAS, ALK and ERBB2 alterations were detected in 22%, 25%, 8.5%, and 2.4%, respectively. EGFR mutations were associated with female sex, Asian race, and never-smoking status; ALK rearrangements were strongly associated with neversmoking status and ERBB2 mutations were strongly associated with Asian race and never-smoking status.92 There is a need to identify predictive biomarkers for targeted therapies. The biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE)-2 clinical study aims to identify biomarkers for optimal patient selection for EGFR, PI3K/AKT and MEK inhibitors.92
Targeted therapy in squamous cell non-small cell lung cancer
Although many of the targeted agents described apply to non-squamous NSCLC, targeted therapy for squamous cell NSCLC is now an area of active clinical research. Characterisation of squamous cell carcinoma by The Cancer Genome Atlas has identified mutations in receptor tyrosine kinase pathways PI3K, AKT and FGFR, which may lead to targeted drugs being developed in the future.93 In addition, the Lung Master Protocol (LUNG-MAP) was recently launched to study the potential for biomarkerdriven targeted therapy for second-line treatment of patients with squamous-cell lung cancer.94
Immunotherapy
Recently, immune checkpoint inhibitors (e.g. anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4): anti- programmed death-1 (PD-1): and anti-PD-L1 have emerged as new therapeutic options. In a phase II study, ipilimumab in combination with first-line chemotherapy showed promise in treating patients with metastatic NSCLC.95 In another phase II study, tremelimumab did not demonstrate superiority over best supportive care in NSCLC patients but a partial response rate was seen in 4.8%, suggesting that tremelimumab warrants further investigation.96
In 2015, the FDA, and in 2016, the EMA, approved the anti-PD1 agent nivolumab for the treatment of non-squamous NSCLC. In Europe, the regulators noted the higher risk of death in patients with aggressive disease,97 Approval was based on data from the phase III CheckMate 057 trial, which found that nivolumab improved OS compared with docetaxel (12.2 months versus 9.4 months) in the second-line treatment of nonsquamous cell NSCLC.98,99 However, CheckMate 026, a study investigating nivolumab in the first-line treatment setting, did not meet its primary endpoint: the PFS was 4.2 months with nivolumab compared with 5.9 months with chemotherapy.100
Pembrolizumab has also received accelerated FDA-approval for the second line treatment of NSCLC following data from the KEYNOTE clinical trials.101,102 Recent long-term data from KEYNOTE-001 (median follow-up duration 23.1 months) reported an OS of 22.1 months for treatment-naive patients and 10.6 months for previously treated patients. The survival benefit increases with increasing PD-L1 positivity.103 In the recently published KEYNOTE-024 study, pembrolizumab was associated with significantly longer progression-free (10.3 months versus 6.0 months) and OS (80.2% at 6 months versus 72.4%) and with fewer AEs compared with platinum-based chemotherapy.104 The KEYNOTE-042 study is also investigating pembrolizumab in the first-line setiing.105
Anti-PD-L1 agents including atezolizumab, durvalumab (MEDI4736),106 and avelumab (MSB0010718C).107,108 are also being developed for NSCLC. In the phase II POPLAR trial, atezolizumab significantly improved OS and overall response rates versus docetaxel in patients with non-squamous and squamous NSCLC with strong PD-L1 expression.109,110 In the phase II BIRCH trial, atezolizumab showed an overall response rate of up to 27% in patients with strong PD-L1 expression.111 Recently presented data from the OAK trial showed superior survival (13.8 months versus 9.6 months) for atezolizumab versus docetaxel in previously treated patients with NSCLC.112 In October 2016, atezolizumab was approved by the FDA for the treatment of patients with metastatic NSCLC whose disease progressed during or following platinumcontaining chemotherapy.
Immunotherapeutic approaches are also in development for squamous cell NSCLC. Nivolumab was approved for use in squamous NSCLC in March 2015 following data from the phase III CheckMate 017 trial, in which nivolumab significantly improved over docetaxel (9.2 months versus 6 months) in the second-line setting in patients with squamous cell NSCLC.113 In a phase II study of nivolumab in refractory patients (2 or more previous lines of treatment) with squamous cell NSCLC, CheckMate 063, the response rate was only 14.5%. However, almost all responders had ongoing responses at study end (median duration of response not reached).114
Combined therapeutic approaches involving immunotherapy are also a promising approach. Ipilimumab has shown efficacy in treating patients with metastatic NSCLC in combination with first-line chemotherapy in one phase II trial.95 The combination of durvalumab and tremelimumab, has demonstrated antitumor activity in patients with locally advanced or metastatic NSCLC.115 In addition, KEYNOTE-189 is investigating the combination of pembrolizumab (MK-3475) and platinum-pemetrexed chemotherapy.116
There is a need to identify patients who are mt likely to benefit from immunotherapy. The expression of PD-1 ligands (PD-L1 or PD-L2) on the surface of tumour cells or immune cells has been shown to be predictive of response to PD-1 blockade in some but not all cases so it cannot be considered a definitive biomarker.117 Whole-exome sequencing has detected a genetic basis for benefit from CTLA-4 blockade in melanoma,118 and from anti-PD-1 therapy in NSCLC.119 Somatic mutations arising from mismatch-repair defects have also been found to predict clinical benefit of immune checkpoint blockade with pembrolizumab.120
Chemotherapy
Despite advances in targeted therapies and immunotherapy, chemotherapy involving a platinum agent combined with a thirdgeneration therapeutic, most commonly taxanes, gemcitabine, vinorelbine or pemetrexed,121 remains central to the treatment advanced NSCLC. Research into new chemotherapeutic agents appeared to have
reached a plateau. However, in 2012, the FDA approved nab-paclitaxel, a nanoparticle albumin-bound (nab) formulation of paclitaxel which, when combined with carboplatin, significantly improved overall response rate in a phase III trial.122 In the phase III PARAMOUNT trial, pemetrexed continuation maintenance therapy is well-tolerated and gave significant improvement in OS (13.9 months versus 11.0 months) compared with placebo in advanced nonsquamous NSCLC.123
As mentioned above, the combination of chemotherapy and targeted therapies/immunotherapies is an area of active clinical research.
Radiotherapy
Stereotactic body radiotherapy is effective in stage 1 NSCLC.124 in advanced NSCLC whole brain radiotherapy (WBRT) and dexamethasone are widely used to treat brain metastases. However, the recent Quality of Life after Treatment for Brain Metastases (QUARTZ) study found that WBRT provides little benefit in this patient group,125 Combined immunotherapy and radiotherapy is also under clinical investigation; ionizing radiation elicits inflammatory signals that may activate tumour-specific T cells.126 A small study found that radiotherapy combined with ipilimumab produced an immune-mediated effect in a patient with NSCLC.127
Conclusions
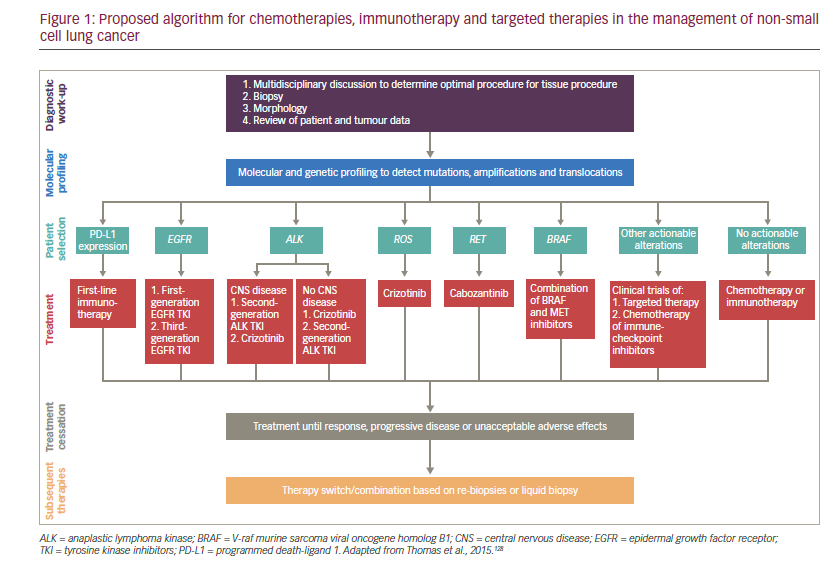
The treatment landscape for NSCLC has expanded greatly in the last decade, and the availability of immunotherapy and therapies targeted towards a specific driver mutation has allowed treatment to be tailored to the patient. Outcomes may be improved by correctly profiling the tumour and using this to inform the appropriate sequence of treatments. There may be a way to either cycle or combine treatments to prevent cancer cells from adapting to specific drugs. However, few biological and clinical data are available to direct the sequencing of immunotherapies and targeted drugs, though an algorithm has recently been proposed by Thomas et al. (see Figure 1).98 More progress also remains to be made towards personalised treatment and shift away from the ‘one-size-fits-all’ approach. Identification of the specific molecular alterations that contribute to the response to targeted therapy will become an important part of selecting appropriate therapies. Reliable biomarkers to predict which patients might benefit from targeted therapy and immunotherapy are also urgently needed. The few treatment advances achieved so far for lung cancer patients with metastatic squamous cell carcinoma contrasts with the progress seen in NSCLC, highlighting another unmet need for improved treatment options for this group of patients.99













