Colorectal cancer is the third most common cancer worldwide and the fourth most common cause of death, with 1.8 million new cases and 881,000 deaths in 2018.1 Rectal cancers account for 28% of these cancers.2 Most colorectal cancers arise from adenomatous polyps and progress to adenocarcinoma through the three major genetic pathways of chromosomal instability, microsatellite instability, and CpG island methylation.3 Locally advanced rectal cancer is often defined as T3, T4, or lymph node-positive disease, and the treatment paradigm has evolved over time from surgery alone, which resulted in high rates of locoregional recurrences, particularly in the absence of total mesorectal excision (TME),4–6 to the current standard of care—neoadjuvant chemoradiotherapy (CRT) followed by TME.7,8 While surgery remains the main component for the curative treatment for locally advanced rectal cancer, nonoperative management is a strategy that is currently being investigated in prospective trials. The purpose of this review is to summarize data on the evolution of management for locally advanced rectal cancer as well as to comment upon emerging evidence for nonoperative management.
Early treatment for rectal cancer
Developed in 1908, the first effective treatment for rectal cancer was the abdominoperineal resection (APR).9 Although procedural mortality rates were high, the operation not only addressed the need to remove the primary rectal cancer, but also recognized the importance of regional node drainage. Subsequent technical refinements in the procedure, and improved perioperative care, reduced both operative morbidity and mortality. With the recognition that lymphatic drainage from rectal cancers was cephalad and progressed retrograde to the perineum only when bulky tumors obstructed proximal lymphatic flow, the sphincter-sparing procedure known as low anterior resection (LAR) became more common.10 More recently, an emphasis on TME has improved locoregional disease control rates significantly due to en bloc resection of the mesorectum, which contains the perirectal lymph nodes, the first echelon of rectal lymph node drainage.11
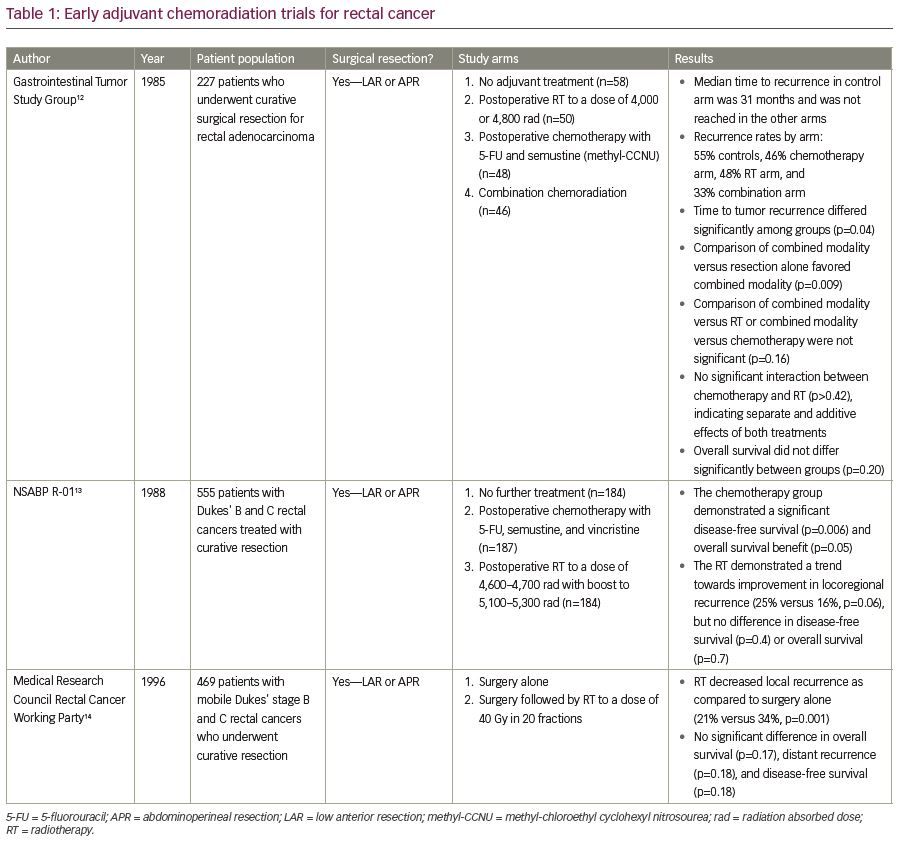
Moving into the 1990s, the primary modality for rectal cancer treatment was surgery followed by adjuvant radiotherapy (RT). Several randomized trials have examined this treatment approach, as summarized in Table 1. For example, in the Gastrointestinal Study Group prospective trial, patients who had undergone either LAR or APR for locally advanced rectal adenocarcinoma were randomized to receive no adjuvant therapy, postoperative RT, postoperative chemotherapy, or a combination of postoperative CRT.12 With a median follow-up of 80 months, overall survival (OS) was not significantly different among the treatment groups (p=0.20), but the local recurrence (LR) rate was highest in the patients who received no adjuvant therapy and lowest in those who received adjuvant CRT (55% versus 33%).12 The median time to recurrence in the control arm that received no adjuvant therapy was 31 months, and the median time to recurrence was not reached in the other arms. Nevertheless, the time to tumor recurrence differed significantly among the four treatment groups (p<0.04) and was significantly prolonged by combined CRT as compared with resection alone (p<0.009).12 It is important to note, however, that TME was not routinely performed at the time of this seminal study. Thus, this study may have resulted in higher LR rates than could be achieved with TME-based operations.
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) R-01 protocol, 555 patients with Dukes’ B and C rectal cancers treated by either LAR or APR were randomized to no further treatment, postoperative adjuvant chemotherapy or postoperative RT (Table 1).13 When compared with surgery alone, the chemotherapy group had improved disease-free survival (DFS) and OS (p=0.006 and p=0.05, respectively), though the benefit appeared to be restricted to males and younger patients.13 When the radiation group was compared with surgery alone, RT showed a trend towards improved locoregional control (16% versus 25%, respectively for locoregional recurrence, p=0.06), but no difference in DFS or OS.13
The Medical Research Council Rectal Cancer Working Party randomized 469 patients to surgery alone by either LAR or APR versus surgery, followed by adjuvant RT to a dose of 40 Gy in 20 fractions. At 5 years, adjuvant RT decreased LR as compared with surgery alone (21% versus 34%, p=0.001), without any significant increase in serious late bowel complications, but there were no significant differences in OS, distant recurrence, and DFS.14 In summary, these trials demonstrated that while postoperative RT may improve local control, adjuvant chemotherapy may provide a survival benefit.
Postoperative chemoradiotherapy
In the 1990s, the National Cancer Institute Consensus Conference concluded that the standard postoperative treatment for patients with rectal cancer with pT3 or N1-2 disease should be CRT.15 Several subsequent trials shaped both type and route of chemotherapy based on providing the optimal benefit. In the US GI Intergroup 86-47-51 trial, 660 patients with stage II or III rectal cancer who had undergone curative surgery with either LAR or APR were randomized to four arms, as shown in Table 2.16 With a median follow-up of 46 months, patients who received protracted venous infusion 5-fluorouracil (PVI 5-FU; continuous infusion) had a significant decrease in the overall rate of local tumor relapse from 47 to 37% (p=0.01) and distant metastasis from 40 to 31% as compared with patients who received bolus 5-FU (p=0.03). Additionally, there was no benefit in patients who received semustine (methyl-chloroethyl cyclohexyl nitrosourea) plus 5-FU. Patients who received a PVI of 5-FU also had a significant improvement in the time to relapse from 53 to 63% at 4 years (p=0.01) and an improvement in OS from 60 to 79% (p=0.0005).16 This study has shaped the modern standard of care in giving radiosensitizing continuous infusion 5-FU during RT.
The successor trial, Intergroup (INT)-0114 was a four-arm trial in which 1,695 patients with T3/4 or node-positive rectal cancer were randomized to the following groups: (1) RT and bolus 5-FU, (2) RT and bolus 5-FU with leucovorin, (3) RT and bolus 5-FU with levamisole, or (4) RT and bolus 5-FU with leucovorin and levamisole, as shown in Table 2.17 With a median follow-up of 7.4 years, there was no difference in OS or DFS by drug regimen.17 However, patients with high-risk T3 node-positive or T4N0 disease had a lower survival rate (45%) compared with low-risk T1-2 node-positive or T3N0 disease (70%). LR did not vary by treatment type, but low-risk patients had a failure rate of 9% compared with 18% for high-risk patients at 5 years (p<0.0001).17
Finally, the INT-0144 trial was a three-arm trial in which 1,917 patients were randomized to either: (1) bolus 5-FU in two cycles, before and after RT, to a dose of 45 Gy with a minimum boost of 5.4 Gy with concurrent PVI 5-FU; (2) PVI 5-FU before, after, and during RT; or (3) bolus 5-FU–leucovorin–levamisole before, after, and during RT (Supplementary Figure 1).18 With a median follow-up of 5.7 years, there was no difference in OS or DFS among the groups and locoregional recurrence was low. However, grade 3 or grade 4 hematologic toxicity was significantly lower in the PVI arm when compared with any other arms of the study (4% versus 49–55%). In summary, these trials demonstrated that PVI 5-FU was the optimal choice for concurrent chemotherapy with postoperative RT in patients with rectal cancer.
Preoperative radiation versus preoperative chemoradiotherapy
Two major trials established the superiority of preoperative CRT over preoperative RT alone. In a trial by Gérard et al., 733 patients with resectable, locally advanced rectal cancer were randomized to either preoperative RT alone at a dose of 45 Gy, or preoperative CRT with bolus 5-FU and leucovorin during weeks 1 and 5, with surgery planned 3–10 weeks afterwards. In both arms, 50% of patients received adjuvant 5-FU.19 While acute toxicity was greater in the CRT arm (14.6% versus 2.7%; p<0.05), LR was lower with CRT (8.1% versus 16.5%; p<0.05). There was no difference in sphincter preservation or 5-year OS.19 Additionally, EORTC 22921 randomized 1,011 patients with clinical stage T3 or T4 resectable rectal cancer to preoperative RT to a dose of 45 Gy, preoperative CRT to a dose of 45 Gy with two cycles of 5-FU and leucovorin, preoperative RT and four cycles of postoperative chemotherapy; or preoperative CRT and postoperative chemotherapy.20 The compliance rates for preoperative chemotherapy were 82.0% versus 42.9% for postoperative chemotherapy. The 5-year OS for all four groups was 65.2% and there was no significant difference between the groups that received chemotherapy preoperatively or postoperatively (p=0.84 and p=0.12, respectively).20 However, the 5-year LR rates were 8.7%, 9.6% and 7.6% in the groups that received chemotherapy preoperatively, postoperatively, or both preoperatively and postoperatively, respectively, versus 17.1% in the group that did not receive chemotherapy (p=0.002).20 Thus, in both of these trials, the addition of chemotherapy to radiation significantly improved local control rates.
Preoperative versus postoperative chemoradiotherapy
The seminal German CAO/ARO/AIO-94 Rectal Cancer Study Group established preoperative CRT as the current standard for treating locally advanced rectal cancer. As shown in Supplementary Figure 1, 827 patients with T3, T4, or node-positive disease were randomized to neoadjuvant CRT followed by TME, or TME followed by adjuvant CRT, with bolus 5-FU rather than PVI 5-FU. Preoperative CRT not only decreased LR at 10 years (7% versus 10%, p=0.048), but also increased the likelihood of sphincter-preservation surgery in those initially felt to require an APR (39% versus 19%, p=0.004). Moreover, preoperative CRT decreased the incidence of acute grade 3–4 toxicity (27% versus 40%, p=0.001) and late grade 3–4 toxicity (14% versus 24%, p=0.01). However, there were no differences in distant recurrence, OS or DFS.8
The NSABP R-03 trial also evaluated preoperative versus postoperative CRT for patients with locally advanced rectal cancer (Supplementary Figure 2). The 5-year DFS was significantly better in the preoperative CRT group (64.7% versus 53.4%, p=0.011) and OS trended towards significance (74.5% versus 65.6%, p=0.065). Among patients who received preoperative therapy, 15% achieved a pathologic complete response (pCR), none of which developed recurrence at last follow-up. Of note, the study accrued only 267 of the 900 planned patients, thereby limiting the statistical power to detect differences.21
Finally, the UK Medical Research Council evaluated short-course preoperative RT versus initial surgery with selective postoperative CRT only in patients with involvement of the circumferential resection margin (CRM; Supplementary Figure 3).22 With a median follow-up of 4 years, patients who received preoperative therapy compared with selective postoperative treatment had significantly lower 3-year LR rates (4.4% versus 10.6%, p<0.0001) and higher 3-year DFS (77.5% versus 71.5%, p=0.013), but there was no difference in OS.22 The aforementioned trials provide substantial evidence favoring preoperative CRT in patients with locally advanced
rectal cancer.

Optimal regimen of preoperative chemotherapy
Several trials have compared 5-FU or capecitabine-based regimens and the addition of oxaliplatin in an attempt to determine the optimal preoperative chemotherapy. In the STAR-01 trial, 747 patients with resectable locally advanced rectal cancer were randomized to receive 50.4 Gy of radiation with either infused 5-FU alone or 5-FU and oxaliplatin.23 While the pCR rate in both arms was 16% and there was no difference in the rate of APR, grade 3 and 4 toxicity was higher in the group that received oxaliplatin (24% versus 8%, p<0.001).23 Another trial that assessed the impact of adding oxaliplatin to preoperative CRT regimens was the ACCORD 12/0405 PRODIGE 2 trial. Specifically, 598 patients with locally advanced rectal cancer were randomized to receive neoadjuvant CRT with either 45 Gy and concurrent capecitabine or 50 Gy with capecitabine and oxaliplatin (CAPOX).24 Similar to the STAR-01 trial, there was no difference in pCR between the groups, but the CAPOX group experienced higher rates of grade 3 and 4 toxicity (25% versus 11%, p<0.001).24 Moreover, at 3 years, there was no significant difference in LR, OS, or DFS between the capecitabine and CAPOX groups (6.1% versus 4.4%, 87.6% versus 88.3%, and 67.9% versus 72.7%, respectively).25 Given that the aforementioned trials demonstrated increased toxicity without benefit in clinical outcomes, oxaliplatin is not typically included in preoperative CRT.
Short-course preoperative radiation
The rationale for short-course preoperative RT in locally advanced rectal cancer is based on two major European randomized trials. The Swedish Rectal Cancer Trial randomized patients with resectable rectal cancer to receive either 25 Gy in five fractions followed by surgical resection within a week of RT, or surgery alone (Supplementary Figure 4).26,27 With a median follow-up of 13 years, patients who received preoperative RT had an significantly improved OS (38% versus 30%), an improved cancer-specific survival rate (72% versus 62%), and a decreased incidence of LR (9% versus 26%).26
The Dutch CKVO 95-04 trial also investigated the role of preoperative short-course RT, followed by TME, versus TME alone (Supplementary Figure 5).28 Of the 1,805 eligible patients, those who received RT had an decreased incidence of LR (5% versus 11%), but there was no difference in OS.29 However, in a subset analysis of patients with stage III cancer and negative circumferential margins, there was an improvement in OS for those who received preoperative RT (50% versus 40%, p=0.03).29 Overall, these European trials established short-course radiation as a reasonable approach for preoperative therapy in rectal cancer.
Preoperative short-course radiotherapy versus preoperative long-course chemoradiotherapy
The German Rectal Cancer Trial established long-course CRT as the standard of care for patients with T3/T4 or node-positive disease, while the Swedish and Dutch trials evaluated the role of preoperative short-course RT in patients with clinical T1–3 disease.8,26,28 Subsequent trials have sought to compare these approaches. Bujko et al. randomized patients with clinical T3 rectal cancers to receive either preoperative short-course followed by TME within 7 days or CRT followed by TME within 4–6 weeks, as shown in Supplementary Figure 6. With a median follow-up of 48 months, there were no significant differences in sphincter preservation, crude LR, DFS, and OS. However, early radiation toxicity was significantly increased in the CRT group (18% versus 3%, p<0.001), though there was no significant difference in late toxicity or overall postoperative complications.30
The TROG Intergroup trial similarly compared preoperative short-course RT with preoperative long-course CRT, as shown in Supplementary Figure 7. There were no significant differences in 3-year LR, distant recurrence, relapse-free survival, OS, or late toxicity.31 Bujko et al. also evaluated the efficacy of short-course RT followed by consolidation chemotherapy, versus long-course CRT, in improving local control in advanced rectal cancers in the Polish II trial (Supplementary Figure 8).32 The authors found that OS was significantly increased (73% versus 65%, p=0.046) and preoperative acute toxicity was significantly reduced (75% versus 83%, p=0.006) in the group receiving short-course RT followed by consolidation chemotherapy versus patients who received long-course CRT. There were no significant differences in DFS, R0 resection rates, pCR rates, local failure, distant metastasis, and postoperative and late complications.32 Overall, these data suggest that short-course RT is at least equivalent to long-course CRT and is more cost effective, especially in countries with limited healthcare budgets.
Interval between radiotherapy and surgery
There have been several studies evaluating the impact of timing of surgery on outcomes in rectal cancer. The Stockholm III trial randomized 840 patients with resectable rectal adenocarcinoma without evidence of metastasis to either: short-course RT (25 Gy in five fractions) followed by surgery within 1 week; short-course RT followed by surgery within 4–8 weeks; or long-course RT only (50 Gy in 25 fractions) followed by surgery within 4–8 weeks.33 There were no significant differences in LR or postoperative complications between the three groups. However, in a subgroup analysis of the two short-course arms of the study, the risk of postoperative complications was significantly lower in the patients who received short-course RT followed by a delay in surgery (41% versus 53%, p=0.001).33
While these data suggest that short-course radiation with a delay in surgery is an effective treatment option, these results are difficult to interpret given that the use of neoadjuvant chemotherapy was not reported, less than 20% of patients received adjuvant chemotherapy, the long-course arm did not include concurrent chemotherapy, and centers were allowed to choose to enroll patients on only the short-course arm of the trial.
The GRECCAR-6 trial also evaluated the impact of surgical timing on outcomes in patients with advanced rectal cancer. Specifically, 265 patients with clinical T3 or T4 or node-positive tumors of the middle or lower rectum who had received long-course CRT (45–50 Gy with 5-FU or capecitabine) were randomized to either surgery 7 weeks or 11 weeks after completion of CRT.34 There was no difference between the two groups in the primary endpoint of pCR rate. However, the morbidity was significantly worse in the 11-week group (44.5% versus 32%, p=0.0404) due to a significantly higher rate of medical complications (32.8% versus 19.2%, p=0.0137). Furthermore, patients who received surgical resection at 11 weeks also had a significantly worse quality of mesorectal resection (78.7% versus 90% complete resection, p=0.0156).34 Thus, this study suggests that delaying surgery may be associated with increased morbidity and a more difficult resection, and is longer than the maximum of 8 weeks until surgery in the Stockholm II Study. Further research is needed to clarify the impact of timing on outcome in advanced rectal cancer.
Total neoadjuvant therapy
As previously discussed, neoadjuvant CRT or preoperative short-course RT followed by TME have significantly reduced LR in locally advanced rectal cancer.8,26,28 However, these advances have not translated into improvements in distant recurrence rates, which has increased interest in the role of systemic therapy. Currently, adjuvant chemotherapy follows neoadjuvant CRT or short-course RT and TME in the treatment paradigm for locally advanced rectal cancer, but multiple trials have emerged to clarify the role of chemotherapy and support a total neoadjuvant therapy (TNT) approach in which all RT and chemotherapy are delivered preoperatively.35
Chemoradiotherapy followed by neoadjuvant chemotherapy
One approach of TNT that has been evaluated is CRT followed by neoadjuvant chemotherapy. In a trial by Zampino et al., 51 patients affected by locally advanced rectal cancer received concurrent capecitabine and RT to a dose of 50.4 Gy followed by two cycles of capecitabine until 2 weeks prior to surgery.36 With a median follow-up of 43 months, 18% of patients had a pCR, 12% and 30% were down-staged in T and N, respectively. Additionally, sphincter preservations rates were 62% for patients with tumors <6 cm from the anal verge and 80% for the entire cohort and 5-year DFS was 85.4% (95% confidence interval [CI]: 75.3–95.4%).36 Another study by Zhu et al. also demonstrated efficacy with minimal toxicity with a TNT approach. Specifically, 42 patients with locally advanced rectal cancer received concurrent CAPOX and radiation to a dose of 44 Gy followed by one cycle of capecitabine with surgery scheduled 6 weeks after completion of neoadjuvant therapy. All patients completed neoadjuvant therapy, with the majority experiencing only mild grade 1 or 2 toxicities.37 Of the entire cohort, six patients achieved pCR and 38 patients received surgery. These 38 patients were divided into good responders with tumor regression grade 3–4 and poor responders with tumor regression grade 1–2. Good responders had improved DFS and OS compared with poor responders (81.6% versus 16.8%, p=0.000; 83.9% versus 40.7%, p=0.007).37
Moreover, in a study by Gao et al., 36 patients received concurrent CRT with two cycles of CAPOX with radiation to a total dose of 50 Gy and one additional cycle of chemotherapy after CRT prior to surgery.38 All patients completed concurrent CRT and underwent TME with 75% of patients undergoing an additional sphincter-sparing surgery, but two patients were unable to complete the additional cycle of chemotherapy due to grade 3 leukopenia and diarrhea.38 A pCR, near pCR, and minimal regression was achieved in 36.1%, 44.4%, 19.5%, respectively and postoperative complications developed in 19.4% of patients.38 Finally, in a phase II trial by Garcia-Aguilar et al., 292 patients with locally advanced rectal cancer received neoadjuvant CRT with 5-FU and 45 Gy with a minimum boost of 5.4 Gy and were divided into four groups: Group 1 (n=60) received TME 6–8 weeks after CRT; Group 2 (n=67) received two cycles of 5-FU, leucovorin and oxaliplatin (mFOLFOX6) between CRT and TME; Group 3 (n=67) received four cycles of mFOLFOX6 between CRT and TME; and Group 4 (n=65) received six cycles of mFOLFOX6 between CRT and TME.39 Of the 259 patients who were analyzed, 18%, 25%, 30%, and 38% of patients in Groups 1, 2, 3, and 4 achieved a pCR, respectively (p=0.0036). Study group was independently associated with pCR with the odds ratio of Group 4 at 3.49 compared with Group 1 (95% CI: 1.39–8.75; p=0.011).39 The most common grade 3 or higher adverse events associated with the neoadjuvant administration of mFOLFOX6 across groups 2–4 were neutropenia (n=5 in Group 3 and n=6 in Group 4) and lymphopenia (n=3 in Group 3 and n=4 in Group 4).39
Neoadjuvant chemotherapy followed by chemoradiotherapy
Another approach of TNT that has been studied in recent trials is neoadjuvant chemotherapy followed by CRT. In a study by Fernández-Martos et al., 108 patients with locally advanced rectal cancer were randomized to preoperative CRT with CAPOX, and concurrent RT to a dose of 50.4 Gy followed by TME and four cycles of postoperative CAPOX (arm A, n=52) or induction CAPOX followed by CRT and TME (arm B, n=56).40,41 The pCR rates were similar between arm A and arm B (13.5%, 95% CI: 5.6–25.8% and 14.3%, 95% CI: 6.4–26.2%, respectively).40 However, compliance with chemotherapy was lower and toxicity with postoperative chemotherapy was higher in arm A versus arm B (57% versus 94%, p<0.0001 and 54% versus 19%, p=0.0004, respectively).40 With a median follow-up of 69.5 months, there were no significant differences in DFS, OS, LR, or distant metastasis.41 In the CONTRE study by Perez et al., 39 patients with locally advanced rectal cancer received eight cycles of mFOLFOX6 followed by concurrent CRT with capecitabine to a dose of 50.4 Gy and TME 6–10 weeks after CRT.42 Among the patients in the study, 89% completed the aforementioned treatment regimen and 33% had a pCR at the time of surgery (95% CI:18.24–47.76%).42 With a median follow-up of 25.5 months, median progression-free survival was not reached. Two patients had a locoregional recurrence and six patients had a distant recurrence, but overall the regimen was well-tolerated and comparable to the current standard.42
Comparison of total neoadjuvant therapy approaches
While both aforementioned TNT approaches demonstrate promising results in comparison to the current standard of care, the optimal sequencing remains unclear. A recent trial by Fokas et al. compares the approaches to each other. Specifically, 311 patients with stage II or III rectal cancer were assigned to induction chemotherapy with three cycles of 5-FU, leucovorin, and oxaliplatin before 5-FU/oxaliplatin CRT to a dose of 50.4 Gy (group A) or to consolidation chemotherapy after CRT (group B).43 Of the 306 patients analyzed, compliance with the treatment regimen was higher in group B than group A, with 97%, 87%, and 93% receiving full-dose RT, concomitant 5-FU, and concomitant oxaliplatin versus 91%, 78%, and 76% respectively.43 Additionally, CRT-related toxicity was lower (27% versus 37%) and pCR rate was higher in group B patients than group A patients (25% versus 17%).43 Further studies are needed to ultimately determine which approach of TNT translates into improved oncologic outcomes. The rationale for utilizing a TNT approach includes potentially addressing micrometastases at an early stage, reversing diverting ileostomies earlier, decreasing toxicity, and increasing tumor regression.44,45 Ultimately, the ability of TNT to improve tumor regression rates may aid in optimizing selection of patients for nonoperative management.
The role of magnetic resonance imaging in risk stratification of rectal cancer
The optimal management of rectal cancer requires assessment of tumor proximity to the mesorectal fascia in order to facilitate successful surgery, given that involvement of the CRM is highly predictive of LR and OS.46 Traditionally, primary tumors have been assessed with digital rectal exam, endorectal ultrasound, and/or pelvic computed tomography. More recently, however, rectal protocol magnetic resonance imaging (MRI) has been favored as the primary modality of tumor assessment due to its prognostic implications, with several studies demonstrating its ability to predict a curative resection with decreased inter-observer variability than endorectal ultrasound.47,48 In a prospective observational study of rectal cancers, the MERCURY study group assessed 408 patients who underwent MRI of the pelvis with a body coil and a high resolution protocol prior to TME in order to evaluate the accuracy of preoperative staging to predict surgical CRMs. The study revealed that 87% of patients had a clear CRM on histopathology, defined as ≥1 mm between the tumor and the margin, and that the specificity for prediction of a clear CRM by MRI was 92%. In patients for whom MRI predicted a clear CRM and who underwent TME, 94% actually had a clear CRM with an accuracy of prediction of 88%.47 In 5-year follow-up from this study, MRI-involved CRM was the only preoperative staging parameter that remained significant on multivariate analysis for poorer OS, DFS, and LR compared with MRI-clear CRM with rates of 42.2% versus 62.2% (p<0.01), 47.3% versus 67.2% (p<0.05), and 7.1% versus 20% (p<0.05), respectively.48
More recently, MRI has been used to assess pCR rates after neoadjuvant therapy. In a study by Bhoday et al., the sensitivity of assessing pCR with magnetic tumor regression grade—a system established by the Magnetic Resonance Imaging in Rectal Cancer European Equivalence study group—versus endoscopically determined residual mucosal abnormality was compared in patients with rectal adenocarcinoma who received neoadjuvant CRT before surgery, with 143 patients assessed by MRI and 119 patients assessed by residual mucosal abnormality analysis.49 Sixteen patients (13.4%) were assessed to have a pCR by residual mucosal abnormality analysis with a sensitivity of 62.5%. By contrast, 18 patients (12.6%) were assessed to have a pCR by MRI tumor regression grade analysis with a sensitivity of 94%. MRI tumor regression grading was 10 times more likely to identify a pCR than clinical assessment of residual mucosal abnormality with no difference in false positive rates.49 Furthermore, studies evaluating radiomic features in predicting tumor response have also been published and are gaining increasing importance in monitoring patients due to recent efforts to pursue nonoperative management after neoadjuvant therapy.50 Lambregts et al., evaluated the utility of MRI and diffusion-weighted imaging (DWI) in diagnosing tumor regrowth in 72 patients who underwent organ preservation after CRT for rectal cancer.51 After assessment of 440 MRIs, 12 patients were found to have developed regrowth. The sensitivity and specificity for standard MRI were 58% and 98%, respectively, while the sensitivity and specificity for standard MRI with DWI were 75% and 97%, respectively. Although there was an increase in sensitivity and specificity with the addition of DWI, DWI did not result in an overall improvement in diagnostic performance in terms of area under the curve.51 While further investigations regarding the utility of MRI are needed, the aforementioned evidence suggests that it could help stratify risk in patients with rectal cancer, predict success of surgical resection, and/or identify optimal candidates for
organ preservation.
Nonoperative management in locally advanced rectal cancer
Neoadjuvant chemoradiotherapy followed by observation
Currently, TME remains the cornerstone of treatment for rectal cancer. Early data for the nonoperative management of rectal cancer originates from retrospective studies that have evaluated patients who were poor surgical candidates and only received either chemotherapy or CRT alone. In these studies, both local control and survival rates ranged from 20–35%.52,53 However, retrospective and prospective data have emerged from several studies evaluating observation after CRT. In a series by Habr-Gama et al. in 2006, patients with resectable rectal adenocarcinoma were treated with neoadjuvant CRT and those with a complete clinical response were observed with CT scans every 6 months and compared with 21 patients (8.3%) who had an incomplete clinical response and ultimately underwent pT0N0M0 resection as shown in Supplementary Figure 9. With a mean follow-up of 57 months, there was a 3% luminal recurrence rate, 4% distant metastasis rate, and 100% 5-year OS rate.54 In an update of 361 patients, the LR rate was 5% and 5-year OS was 93% in the patients who achieved a complete clinical response.55 In their 2013 series, Habr-Gama et al. again assessed 70 patients for complete response 10 weeks after completing neoadjuvant CRT, as shown in Supplementary Figure 10.56 In this cohort, 47 patients (68%) had an initial complete response and continued monitoring without surgical resection. Of these patients, eight developed regrowth within a year, four developed recurrences after a year, and 39 (57%) sustained a complete clinical response at a median follow-up of 56 months. Of the entire cohort, 50% did not undergo surgery.56
In a study by Maas et al., 192 patients with MRI-staged cT3, cT4 or node-positive rectal cancer underwent neoadjuvant therapy and those who had a complete clinical response—defined as no residual tumor on imaging and endoscopy—were assigned to a wait-and-see policy, as shown in Supplementary Figure 11. With a mean follow-up of 25 months, only one observed patient developed a LR which was salvaged with transanal endoscopic microsurgery (TEM) and the probability of 2-year DFS and OS was 93% and 91%, respectively.57 Appelt et al. also looked at the role of observation in patients with distal rectal cancers in the lower 6 cm of the rectum after neoadjuvant CRT, as shown in Supplementary Figure 12. In their study, 51 patients with distal T2 or T3 rectal cancers received neoadjuvant CRT.58 Forty of these patients achieved a complete clinical response and were monitored, while the rest were referred for surgical resection. With a median follow-up of 23.9 months, LR at 1 year was 15.5%, while sphincter preservation was 72% at 1 year and 69% at 2 years. The most common reported acute toxicity was diarrhea and late toxicity was rectal bleeding.59
Moreover, there have been a couple of smaller single-institution studies further supporting the use of nonoperative management for rectal cancer. For example, in a study by Dalton et al., 49 patients underwent preoperative CRT for rectal cancer and 12 patients (24%) were found to have had a complete response per MRI, as shown in Supplementary Figure 13.59 Of these 12 patients, six progressed and were subsequently referred for surgical resection, while six remained disease-free and avoided surgery with a mean follow-up of 25.5 months.59 In a trial by Smith et al., patients with distal T2, T3, T4, or node-positive disease who received long-course CRT were reviewed. Thirty-two patients who had a clinical complete response (no palpable tumor on exam and no pathology endoscopically) underwent nonoperative management and were compared with 57 patients who had a pCR after neoadjuvant CRT and rectal resection, as shown in Supplementary Figure 14.60 In the observation group, there were six local failures with a median time to failure of 11 months. All these patients were able to be salvaged with surgical resection and remained disease-free for a median time of 17 months. Moreover, the distant DFS and OS were similar for the nonoperative and operative groups.61
The International Wait and Watch Database is one of the largest studies of nonoperative management in rectal cancer. In this study, 880 rectal cancer patients who achieved a complete clinical response after neoadjuvant therapy were observed, as shown in Supplementary Figure 15. With a median follow-up of 3.3 years, the 2-year local regrowth rate was 25.2% with the vast majority of regrowth occurring within the bowel wall. For patients with local regrowth, the 5-year disease specific and OS rates were 84% and 75.4%, respectively, while for patients with a sustained complete clinical response the rates were 97.3% and 87.9%, respectively.61 Overall, while criteria for optimal selection of candidates for nonoperative management are still being investigated, the aforementioned data indicate that observation following neoadjuvant CRT for patients with a complete clinical response is a promising alternative to surgery.
Neoadjuvant chemoradiotherapy followed by limited excision
Organ preservation with either local excision or minimally invasive surgery is another alternative to TME and has been investigated in prospective studies. In a single-arm study by Garcia-Aguilar et al., 72 patients with T2N0 distal rectal cancer were treated with neoadjuvant CRT consisting of CAPOX and 45 Gy, with a boost to 50.4 Gy, followed by local excision.62 With a median follow-up of 56 months, three patients developed LR and five patients developed distant recurrence; six of these patients underwent salvage surgery. For the entire cohort, 3-year DFS and OS was 88% and 95%, respectively.62 GRECCAR 2 was a similar trial, which evaluated the role of local excision in 145 patients with T2 or T3 lower rectal cancer who underwent neoadjuvant CRT consisting of 50 Gy and concomitant CAPOX. Patients with a good clinical response (defined as <2 cm of residual tumor) were randomized to either local excision (n=74) or TME (n=74).63 The primary endpoint was a composite outcome of death, recurrence, morbidity, and side-effects 2 years after surgery; one or more of these composite events occurred in 56% of patients in the local excision group and 48% of the TME group (p=0.43). Twenty-six patients in the local excision group ultimately underwent a TME. There was no significant difference between the groups in any of the components of the composite outcome, and the superiority of local excision over TME was not established.63
The CARTS study by Verseveld et al. further investigated the role of organ preservation in clinically staged T1–3 N0 distal rectal cancer by evaluating the feasibility of TEM as an alternative to TME. Specifically, 55 patients underwent neoadjuvant CRT with 50 Gy and concurrent capecitabine, and were evaluated 6–8 weeks after completion. Patients who had a T0–2 tumor after neoadjuvant therapy underwent TEM and the other patients underwent TME.64 Fifty-one patients were eligible for the study and 47 patients proceeded to TEM. With a median follow‐up of 17 months, there were only four local recurrences, three of which were in patients with ypT2 and one in a patient with ypT1 disease. Ultimately, TEM enabled organ preservation for half of these patients with rectal cancer.64 Overall, these studies suggest that neoadjuvant CRT followed by local excision may be an acceptable alternative to TME, but follow-up data are limited and further studies are necessary to validate these findings.
Ongoing prospective trials of nonoperative management in rectal cancer
There are currently additional ongoing prospective trials investigating the role of nonoperative management in rectal cancer. For example, the Cancer Institute in São Paulo has an ongoing phase II trial (ClinicalTrials.gov Identifier NCT02052921) investigating surgical resection versus observation in patients with middle and distal rectal cancer who achieved a complete clinical response after neoadjuvant CRT with study completion expected in December 2020. Additionally, a phase III trial by Vrije Universiteit Medical Center (NCT02371304) compares TME versus adjuvant CRT after local excision for early stage rectal cancers, with study completion expected in January 2023. These randomized trials are ongoing and will help to elucidate the role of nonoperative management for rectal cancer.
Conclusion
The current standard therapy for locally advanced rectal cancer is neoadjuvant CRT or short-course RT followed by TME and adjuvant chemotherapy. Studies comparing short-course RT versus long-course CRT have demonstrated equivalence, but longer follow-up is still needed. MRI is increasingly being used to determine pCR following neoadjuvant therapy to either predict optimal surgical candidates or as a mechanism for monitoring patients. While TME remains the cornerstone of treatment for locally advanced rectal cancer, nonoperative management is an emerging alternative treatment paradigm for achieving comparable oncologic control with encouraging early results. Current ongoing and future studies will continue to inform the optimal treatment paradigm in patients with locally advanced rectal cancer.