Trifluridine/tipiracil (FTD/TPI) is a novel oral formulation of two drugs with promising results in the treatment of metastatic colorectal cancer (mCRC).1Expand Reference Trifluridine is a thymidine-based nucleoside analogue that, after intracellular phosphorylation, gets incorporated into DNA, causing DNA dysfunction.2Expand Reference It was first identified by Callahan et al. in 1996 as an active impurity in the herbicide trifluralin, which has anti-parasitic properties.3Expand Reference On the other hand, tipiracil, a thymidine phosphorylase inhibitor, was developed by K. Richardson in 1990 as part of a research programme aimed at creating a broad-spectrum anti-fungal agent.4Expand Reference
The rationale for combining trifluridine and tipiracil lies in their synergistic pharmacological effect: tipiracil enhances the bioavailability of trifluridine by inhibiting its breakdown.5Expand Reference Studies have shown that combining trifluridine/tipiracil with an anti-angiogenic agent that targets vascular endothelial growth factor (VEGF) (bevacizumab [BEV]) can lead to augmented intracellular concentrations of active trifluridine, as demonstrated in preclinical studies.6Expand Reference This combination has been extensively studied, with clinical trials reporting promising results regarding the efficacy and safety in patients with refractory mCRC.7Expand Reference
FTD/TPI was developed by Taiho Pharmaceutical (Tokyo, Japan) and was first approved as a novel oral nucleoside anti-tumour combination agent in Japan in March 2014.8Expand Reference It has shown significant survival benefits in patients with refractory mCRC in a randomized phase III trial involving both Asian and non-Asian patients.1Expand Reference In September 2015, it was approved by the US Food and Drug Administration following a phase III clinical trial indicating an overall survival (OS) benefit in patients with refractory mCRC.9Expand Reference The addition of BEV, an established anti-cancer agent that inhibits angiogenesis, was first studied in the C-TASK FORCE trial (ClinicalTrials.gov identifier: NCT02743221) in Japan, followed by another study in Denmark in 2017/2018.10,111011 These studies demonstrated that the combination therapy with BEV was associated with significantly longer median progression-free survival (PFS) and OS compared with FTD/TPI monotherapy.12Expand Reference More recently, the multinational phase III SUNLIGHT (ClinicalTrials.gov identifier: NCT04737187), TAS-CC3 (UMIN000022438) and BiTS (ClinicalTrials.gov identifier: NCT03447490) studies demonstrated that the combination of FTD/TPI and BEV has clinically meaningful activity in previously pre-treated patients with mCRC, establishing it as a potential new treatment option.12–1812131415161718 These studies also highlighted that ongoing clinical trials are investigating this combination in earlier treatment lines for patients with mCRC.
In this review, we will summarize this treatment trajectory and discuss potential approaches to be explored in the future.
Preclinical studies
In vitro experiments with TAS-102 (FTD/TPI), a synergistic combination of trifluorothymidine and the thymidine phosphorylase inhibitor TPI, have shown promising results in preclinical studies, particularly in overcoming resistance to standard chemotherapies such as 5-fluorouracil (5-FU). Initially, the compound was recognized for its dual mechanism of action: the inhibition of thymidylate synthase (TS) and the incorporation of FTD into DNA. The latter mechanism, in particular, was found to be significantly more effective in TAS-102 compared with other anti-tumour nucleosides, correlating positively with its anti-tumour activity in xenograft models.19Expand Reference While the drug’s pharmacokinetics in in vitro settings remain a subject for further exploration, its non-cross-resistance with 5-FU and possible anti-angiogenic effects through thymidine phosphorylase inhibition make it a compelling candidate for both research and future clinical investigations. However, the dual synergistic action of both components improves cytotoxicity by activating different pathways, leading to cell death.
Subsequent studies expanded on these findings, demonstrating TAS-102’s efficacy against a range of 5-FU-resistant cancer types, including colorectal, pancreatic and oesophageal cancers. These studies highlighted the compound’s ability to induce marked DNA fragmentation in tumour cells, a key indicator of its cytotoxic effects, as illustrated in Figure 1.20Expand Reference This cytotoxicity was attributed to the high dosage and short exposure times of TAS-102, which were effective in both in vitro and xenograft models. TAS-102’s unique mechanism and metabolism position it as a promising treatment alternative for patients resistant to or intolerant of 5-FU-based fluoropyrimidines.21,222122 This has the potential to reduce the toxicity of each component, ensuring safety in the use of both entities in improving cytotoxicity while minimizing potential toxicity in each of the entities used alone.
Figure 1: Comparison of the mechanisms of action of TAS-102 and 5-FU20Expand Reference
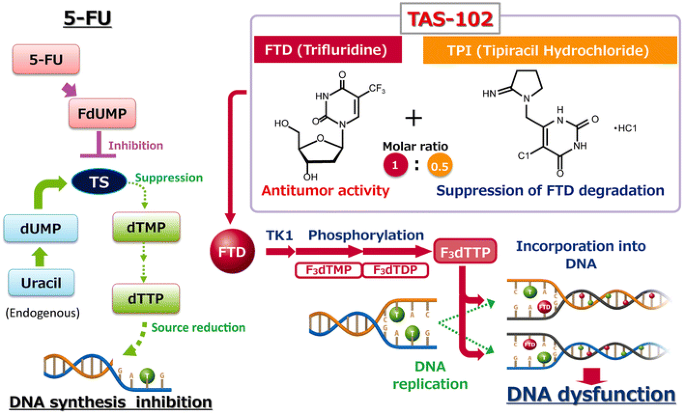
Comparison of the mechanisms of action of TAS-102 and 5-FU
Reproduced and adapted with permission from Longo-Muñoz et al. (2017)20Expand Reference (https://creativecommons.org/licenses/by/4.0/).
5-FU = 5-fluorouracil; dTMP = deoxythymidine monophosphate; dTTP = deoxythymidine triphosphate; dUMP = deoxyuridine monophosphate; F3dTDP = trifluorodeoxythymidine diphosphate; F3dTMP = trifluorodeoxythymidine monophosphate; F3dTTP = trifluorodeoxythymidine triphosphate; FdUMP = fluorodeoxyuridine monophosphate; TK1 = thymidine kinase 1; TPI = tipiracil hydrochloride; TS = thymidylate synthase.
Further exploration of TAS-102’s mechanisms revealed additional therapeutic potentials, such as its anti-angiogenic properties. The compound was shown to inhibit platelet-derived endothelial cell growth factor, which is identical to thymidine phosphorylase, an enzyme involved in tumour angiogenesis and metastasis. This inhibition suggests that TAS-102 could also play a role in reducing liver metastasis in colorectal cancer, a significant factor affecting patient survival.21Expand Reference
Moreover, the role of the tipiracil compound (TPI), a specific inhibitor of thymidine phosphorylase, is crucial for enhancing the bioavailability and efficacy of anti-tumour 2′-deoxyribonucleosides. This selective inhibition has been shown to potentiate the biological activity of these nucleosides in cancer treatment, significantly increasing their blood levels and anti-tumour activity when used in combination, as demonstrated in human stomach cancer xenograft models.23Expand Reference
Collectively, these preclinical studies underscore the multi-faceted mechanisms through which TAS-102 combats cancer, providing a robust foundation for its clinical application in treating resistant forms of the disease. The integration of direct DNA damage, TS inhibition and anti-angiogenic effects, along with the modulation of nucleoside metabolism, presents a comprehensive approach to cancer therapy that could significantly enhance treatment outcomes for patients with resistant tumours.
Clinical studies in metastatic colorectal cancer
Phase I clinical trials
As a newly developed nucleoside for managing refractory mCRC, several clinical studies have been conducted to determine the safety, toxicity and tolerable dosing of the FTD/TPI (TAS-102) combination. Phase I clinical trials have been conducted worldwide and are registered in more than 60 countries, aimed at determining the maximum tolerated dose (MTD), dose-limiting toxicity (DLT) and recommended phase II dose (RP2D).
In 2006, Hong et al. proposed that the initial dose of TAS-102 was 100 mg/m2/day, based on a preclinical monkey model. However, the first two patients experienced substantial toxicity, prompting a lower dose level to be explored. Subsequent dose levels showed certain toxicities, eventually establishing 50 mg/m2/day as the MTD for this schedule.24Expand Reference
In the study by Overman et al., TAS-102 was administered three times daily on days 1–5 and 8–12 every 4 weeks. The trial enrolled 15 patients and identified granulocytopaenia and fatigue as DLTs, with granulocytopaenia being the primary toxicity observed. Although no responses were noted, prolonged stable disease was observed in nine patients.25Expand Reference
Another study by Overman et al. explored TAS-102 administration once daily on a 5-day-per-week schedule, either on days 1–5 and 8–12 every 4 weeks (schedule A) or on days 1–5 every 3 weeks (schedule B). Sixty-three patients participated, with granulocytopaenia again being the predominant DLT. The recommended phase II doses were established as 100 mg/m2/day for schedule A and 160 mg/m2/day for schedule B.26Expand Reference
Doi et al. conducted a dose-escalation study in Japanese patients, administering TAS-102 twice daily on days 1–5 and 8–12 in a 28-day cycle. The study observed DLTs such as severe leukopaenia, neutropaenia and thrombocytopaenia, particularly at the extremes of the dosage range (30 and 70 mg/m2). The RP2D was set at 70 mg/m2/day, with a disease-control rate of 50.0% and a median PFS of 2.4 months among patients with mCRC.27Expand Reference
Overall, these phase I trials of TAS-102 across different patient populations and dosing schedules consistently identified granulocytopaenia as a significant DLT, along with varying degrees of other toxicities such as fatigue, anaemia and gastrointestinal symptoms. These trials helped in establishing dosing recommendations for subsequent studies, with a focus on optimizing the balance between efficacy and tolerability in a heavily pre-treated patient population.
BiTS study (phase Ib/II)
The BiTS study was a phase Ib/II clinical trial conducted at several Japanese hospitals, enrolling patients with mCRC who were refractory or intolerant to standard chemotherapies. The trial aimed to compare the efficacy and toxicity of a biweekly TAS-102 plus BEV regimen with the standard schedule and evaluated it as a potential treatment option with the expectation of equivalent efficacy but with reduced toxicity and a simpler regimen schedule. The primary endpoint was the PFS rate at 16 weeks.15Expand Reference
A total of 46 patients were enrolled, and the RP2D was determined to be 70 mg/m2/day TAS-102. The results showed promising anti-tumour activity, with a 16-week PFS rate of 40.9%, a median PFS of 4.29 months and an OS of 10.86 months. The combination treatment showed an acceptable toxicity profile, with common grade ≥3 adverse events, including hypertension (40.9%), neutropaenia (15.9%) and leukopaenia (15.9%).15Expand Reference
A sub-group analysis showed a connection between chemotherapy-induced neutropaenia (CIN) during the initial two cycles and the prognosis of TAS-102 plus BEV. Specifically, those experiencing CIN had a longer OS compared with those without CIN (hazard ratio [HR]=0.44; 95% confidence interval [CI], 0.2–0.95, p=0.036), as shown in Table 1.15Expand Reference However, even without considering CIN, the combination of biweekly TAS-102 plus BEV showed better results compared with TAS-102 alone, with a median OS of 6.7–7.1 months versus 9.5 months, respectively.15Expand Reference
Table 1: Pre-specified sub-group analysis of factors affecting overall survival15Expand Reference
|
|
Results of full input |
Backward selection (threshold p=0.20) |
|||||||
|
Variables |
HR |
95% CI |
|
p-Value |
HR |
95% CI |
|
p-Value |
|
|
Age |
Per year |
0.96 |
0.93 |
1 |
0.05 |
0.96 |
0.92 |
0.99 |
0.022 |
|
ECOG PS |
PS1 |
3.07 |
1.24 |
7.6 |
0.015 |
2.99 |
1.44 |
6.18 |
0.003 |
|
Tumour location |
Right-sided |
0.91 |
0.36 |
2.27 |
0.833 |
– |
– |
– |
– |
|
RAS status |
Mutant |
1.01 |
0.49 |
2.07 |
0.989 |
– |
– |
– |
– |
|
Prior regorafenib administration |
Yes |
0.64 |
0.26 |
1.58 |
0.329 |
– |
– |
– |
– |
|
Neutropaenia |
Within second cycles |
0.8 |
0.36 |
1.78 |
0.581 |
– |
–– |
– |
– |
Despite mild haematological side effects, the biweekly TAS-102 plus BEV combination demonstrated promising therapeutic effects. This prompts the re-evaluation of the current TAS-102 schedule when combined with BEV. Adopting a biweekly TAS-102 plus BEV regimen could potentially mitigate the need for unnecessary TAS-102 dose reductions, maintain higher doses and potentially prove effective even in cases without CIN.15Expand Reference
Although the study had several limitations, including its non-randomized design, relatively small sample size and the impact on quality of life (QoL) not being evaluated, the biweekly TAS-102 plus BEV showed promising anti-tumour efficacy and acceptable toxicity. These suggest that it could be a treatment option for heavily treated patients with mCRC, especially those who are not eligible for intensive therapy.
TASCO 1 trial (phase II)
The TASCO 1 trial (an open-label, randomized, phase II study comparing S 95005 plus bevacizumab to capecitabine plus bevacizumab in patients with previously untreated metastatic colorectal cancer who are non-eligible for intensive therapy; ClinicalTrials.gov identifier: NCT04012207) was a randomized, non-comparative phase II trial conducted at 52 participating centres in several European countries and in Australia and Brazil. The study included patients aged 18 years or older with a recent diagnosis of mCRC, known as the rat sarcoma virus mutation status, who had not received systemic anti-cancer therapy and were not eligible for intense chemotherapy. In this trial, patients were randomized in a 1:1 ratio to receive either trifluridine/tipiracil plus BEV (TT-B) or capecitabine plus BEV (C-B).28Expand Reference
The primary focus of the study was OS, and findings, as of 1 September 2020, revealed a median OS of 22.3 months with TT-B compared with 17.7 months with C-B and the adjusted HR for OS was 0.78 (95% CI, 0.55–1.10). It was noted that no variables negatively affected OS with TT-B, and the safety results were consistent with prior findings.28Expand Reference
Regarding the reported adverse events, hand–foot syndrome occurred in 52.6% of patients in the C-B arm, with 11.8% experiencing severe cases (grade ≥3). Conversely, this condition was much less common in the TT-B arm, occurring in only 3.9% of patients. However, the TT-B arm experienced a higher incidence of haematological treatment-emergent adverse events (TEAEs), including grade 3 or 4 neutropaenia, anaemia and thrombocytopaenia. Serious TEAEs were reported in 66.2% and 59.2% of patients in the TT-B arm and C-B arm, respectively. Additionally, the percentage of patients discontinuing study medication due to a TEAE was similar between the groups: 42.9% in TT-B and 42.1% in C-B.28Expand Reference
RECOURSE trial (phase III TAS-102)29Expand Reference
In a comprehensive phase III clinical trial, Mayer et al. investigated the efficacy and safety of TAS-102, a novel oral medication combining trifluridine and tipiracil hydrochloride, for the treatment of refractory colorectal cancer.1Expand Reference This global study was a double-blinded, randomized, placebo-controlled study involving 800 patients from Japan, the USA, Europe and Australia, randomly assigned in a 2:1 ratio to receive TAS-102 versus placebo, respectively (ClinicalTrials.gov identifier: NCT01966306).29Expand Reference It included patients aged 18 years and older with adequate liver, bone marrow and renal functions with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 who had biopsy-confirmed adenocarcinoma of the colon or rectum and additionally with evidence of tumour progression within 3 months after the last administration of chemotherapy.1Expand Reference
The study’s primary outcome was OS, defined as the time from randomization to death from any cause. Secondary endpoints included PFS, objective response rate, rate of disease control and safety. The median OS was 7.1 months for the TAS-102 group versus 5.3 months for the placebo group, respectively, with a 1-year OS rate of 27% for patients treated with TAS-102 and 18% for those treated with placebo.1Expand Reference
The findings from the trial indicated a significant improvement in survival outcomes for patients treated with TAS-102. Specifically, the median OS for patients receiving TAS-102 was 7.1 months compared with 5.3 months for those receiving the placebo, as shown in Table 2.1Expand Reference This represented a 32% reduction in the risk of death for the TAS-102 group, with an HR of 0.68 (95% CI, 0.58–0.81; p<0.001).1Expand Reference
Table 2: Main findings from the RECOURSE trial (phase III) of TAS-102 in refractory metastatic colorectal cancer1Expand Reference
|
Outcome |
TAS-102 group |
Placebo group |
Hazard ratio (95% CI) |
p-value |
|
Median overall survival (months) |
7.1 |
5.3 |
0.68 (0.58–0.81) |
<0.001 |
|
Median time to worsening performance status (months) |
5.7 |
4.0 |
0.66 (0.56–0.78) |
<0.001 |
|
Neutropaenia, any grade (incidence %) |
67 |
<1 |
– |
– |
|
Leukopaenia, any grade (incidence %) |
77 |
5 |
– |
– |
|
Febrile neutropaenia, any grade (incidence %) |
4 |
– |
– |
– |
|
Deaths related to TAS-102 |
1 |
– |
– |
– |
Data were extracted from Mayer et al., 2015.1Expand Reference
CI = confidence interval.
However, the use of TAS-102 was associated with higher rates (grade >3) of certain adverse events. Notably, 38% of patients treated with TAS-102 developed neutropaenia and 21% developed leukopaenia.1Expand Reference
Overall, the phase III trial conducted by Mayer et al. provided strong evidence that TAS-102 offers a significant survival benefit for patients with refractory colorectal cancer, despite an increased risk of certain haematological toxicities. These findings support the potential of TAS-102 as a valuable treatment option in this patient population, contributing to both extended survival and prolonged maintenance of functional status.
SUNLIGHT trial (phase III TAS-102 plus bevacizumab)
In the study conducted by Prager et al., the efficacy and safety of combining FTD/TPI with BEV for the treatment of refractory mCRC were evaluated. This research is significant, as it builds on previous findings revealing that FTD/TPI alone can prolong OS in this patient population. The study aimed to determine if adding BEV to the treatment regimen could further enhance survival outcomes.12Expand Reference
The trial involved 492 adults, who were equally divided into two groups: one received the combination of FTD/TPI plus BEV and the other received FTD/TPI alone in the treatment of advanced metastatic CRC worldwide. The trial included participants who had received no more than two previous chemotherapy treatments for colorectal cancer with distant metastasis. This chemotherapy included but was not limited to fluoropyrimidine, irinotecan, oxaliplatin and neoadjuvant or adjuvant therapy. FTD/TPI was administered orally twice daily from days 1 to 5 and from 8 to 12 of every 28-day cycle, while BEV was given as an intravenous injection on days 1 and 15 of every 28-day cycle. The primary endpoint of the study was OS, and the secondary endpoints were PFS and safety measures, particularly focusing on the time to worsening of the ECOG performance status score.12Expand Reference
The results were promising, as summarized in Table 3, which presents the main outcomes obtained from this study.12Expand Reference
Table 3: Comparing the main outcomes of the study on the efficacy of TAS-102 alone versus TAS-102 plus bevacizumab in treating refractory metastatic colorectal cancer12Expand Reference
|
Outcome metric |
FTD/TPI alone group |
FTD/TPI + bevacizumab combination group |
Hazard ratio |
|
Median overall survival |
7.5 (95% CI, 6.3–8.3) |
10.8 (95% CI, 9.4–11.8) |
For death, 0.61 (95% CI, 0.49–0.77), p<0.001 |
|
Median progression-free survival (months) |
2.4 (95% CI, 2.1–3.2) |
5.6 (95% CI, 4.5–5.9) |
For disease progression or death, 0.44 (95% CI, 0.36–0.54), p<0.001 |
|
Median time to worsening of the ECOG performance status score (months) |
6.3 (95% CI, 5.6–7.2) |
9.3 (95% CI, 8.3–10.6) |
For worsening ECOG score, 0.54 (95% CI, 0.43–0.67) |
Data were extracted from Prager et al., 2023.12Expand Reference
CI = confidence interval;ECOG = Eastern Cooperative Oncology Group;FDP/TPI = trifluridine/tipiracil.
Safety profiles were manageable in both groups, and the most common adverse events were neutropaenia, nausea and anaemia, as shown in Table 4.12Expand Reference Importantly, no treatment-related deaths were reported. Additionally, the combination therapy also delayed the worsening of the ECOG performance status score, with a median time of 9.3 months compared with 6.3 months in the FTD/TPI group.12Expand Reference
Table 4: Adverse events of any cause occurring in at least 10% of the patients in the group that received trifluridine/tipiracil plus bevacizumab during the treatment period12Expand Reference
|
Event |
FTD/TPI plus bevacizumab (n=246) |
FTD/TPI (n=246) |
||
|
|
Any grade |
Grade 3 or 4 |
Any grade |
Grade 3 or 4 |
|
|
Number of patients (%) |
|||
|
Neutropaenia |
153 (62.2) |
106 (43.1) |
126 (51.2) |
79 (32.1) |
|
Nausea |
91 (37.0) |
4 (1.6) |
67 (27.2) |
4 (1.6) |
|
Anaemia |
71 (28.9) |
15 (6.1) |
78 (31.7) |
27 (11.0) |
|
Asthenia |
60 (24.4) |
10 (4.1) |
55 (22.4) |
10 (4.1) |
|
Fatigue |
53 (21.5) |
3 (1.2) |
40 (16.3) |
9 (3.7) |
|
Diarrhoea |
51 (20.7) |
2 (0.8) |
46 (18.7) |
6 (2.4) |
|
Decreased appetite |
50 (20.3) |
2 (0.8) |
38 (15.4) |
3 (1.2) |
|
Vomiting |
46 (18.7) |
2 (0.8) |
36 (14.6) |
4 (1.6) |
|
Thrombocytopaenia |
42 (17.1) |
7 (2.8) |
28 (11.4) |
3 (1.2) |
|
Decreased neutrophil count |
34 (13.8) |
22 (8.9) |
17 (6.9) |
13 (5.3) |
|
Abdominal pain |
29 (11.8) |
5 (2.0) |
27 (11.0) |
4 (1.6) |
|
Constipation |
27 (11.0) |
0 |
28 (11.4) |
2 (0.8) |
|
Stomatitis |
27 (11.0) |
1 (0.4) |
9 (3.7) |
0 |
|
Hypertension |
25 (10.2) |
14 (5.7) |
5 (2.0) |
3 (1.2) |
Data are included for all the patients who received at least one dose of trial.
Adapted from Prager et al., 2023.12Expand Reference
FTD/TPI = trifluridine/tipiracil.
The sub-group analysis of this study indicated that the benefit of the combination therapy over FTD/TPI alone was consistent across various sub-groups. This includes sub-groups defined by age, sex, geographic region, the ECOG performance status score and prior exposure to anti-VEGF therapies (not necessarily BEV). The HRs for OS and PFS consistently favoured the combination therapy in these sub-groups, with no significant interaction between sub-group characteristics and treatment effects.12Expand Reference
In conclusion, this study provided compelling evidence that the combination of FTD/TPI with BEV significantly enhances both OS and PFS in patients with refractory mCRC, with statistical significance indicated by p-values <0.001. The safety profile of this combination therapy was manageable, with neutropaenia, nausea and anaemia being the most common adverse events, and no treatment-related deaths were reported. In addition, the combination therapy notably delayed the worsening of the ECOG performance status score, suggesting an extension of survival and an improvement in the QoL for these patients. Notably, this study stands out as the most important of this review, highlighting a promising treatment option that could significantly enhance the QoL and survival outcomes for this challenging patient population.
Ongoing studies and future endeavours of TAS-102 plus bevacizumab in metastatic colorectal cancer
Building on the promising findings from the SUNLIGHT study, ongoing clinical trials are actively exploring the combination of TAS-102 (trifluridine/tipiracil) and BEV for refractory mCRC, as presented in Table 5.30–38303132333435...38
Table 5: Ongoing studies with TAS-102 plus bevacizumab in metastatic colorectal cancer30–38303132333435...38
|
Identifier |
Phase |
Treatment |
Primary endpoint |
|
NCT0603920230Expand Reference |
II |
CA102N combined with TAS-102 compared with bevacizumab combined with TAS-102 |
PFS |
|
NCT0620200131Expand Reference |
I/II |
Irinotecan, trifluridine/tipiracil (TAS-102) plus bevacizumab |
ORR |
|
NCT0583995132Expand Reference |
Cohort |
Regorafenib, trifluridine/tipiracil (TAS-102) plus bevacizumab |
Descriptive analysis |
|
NCT0468396533Expand Reference |
II |
Pemetrexed, trifluridine/tipiracil (TAS-102) plus bevacizumab |
ORR |
|
NCT0585449834Expand Reference |
II |
Liposomal irinotecan, trifluridine/tipiracil (TAS-102) plus bevacizumab |
PFS |
|
NCT0500713235Expand Reference |
II |
Panitumumab, trifluridine/tipiracil (TAS-102) plus bevacizumab |
ORR |
|
NCT0606070436Expand Reference |
II |
Envafolimab, trifluridine/tipiracil (TAS-102) plus bevacizumab |
ORR |
|
NCT0580693137Expand Reference |
II |
Oxaliplatin, irinotecan, trifluridine/tipiracil (TAS-102) plus bevacizumab |
DCR |
|
NCT0624206738Expand Reference |
II |
Trifluridine/tipiracil (TAS-102) plus bevacizumab |
PFS |
Source: ClinicalTrials.gov.
DCR = disease control rate;ORR = objective response rate;PFS = progression-free survival.
One notable ongoing trial is a phase I/II study that combines irinotecan, TAS-102 and BEV. This trial targets patients with mCRC who have already undergone standard therapies. The study was initiated on 1 October 2022 and is expected to be completed by 30 September 2026. It aims to assess the safety and efficacy of this combination as a second-line treatment, potentially providing a new option for patients with limited alternatives.39Expand Reference
Another important study involves the combination of pemetrexed, TAS-102 and BEV. This exploratory study targets patients who have progressed after standard second-line therapy for mCRC. The study was initiated on 1 January 2021 and is expected to be completed by 2024. Its goal is to evaluate the safety, efficacy and optimal dosing regimens of this combination therapy.40Expand Reference
Additionally, the STAR-T study (ClinicalTrials.gov Identifier: NCT05839951) is an observational study examining sequential treatment with regorafenib followed by TAS-102, with or without BEV, in patients with mCRC. This study, which was initiated on 24 April 2023, is expected to provide real-world outcomes from community clinical practices in the USA, being currently active.41Expand Reference
Future research endeavours are likely to focus on several key areas. Expanding the patient population eligible for the combination therapy of TAS-102 and BEV is a priority. This could include patients at different stages of the disease and those who have received various prior treatments. Moreover, combining TAS-102 and BEV with other novel agents, such as immune-checkpoint inhibitors, could further enhance treatment efficacy and patient outcomes. It is important to note that immune cell programmed cell death ligand-1 expression is significantly higher in mismatch repair-deficient (MSI-H: microsatellite instability-high) colorectal cancer compared with mismatch repair-proficient (MSI-L: microsatellite instability-low) tumours. Given this, the combination of these therapies could be especially beneficial for MSI-H tumours. However, challenges remain in accurately assessing the MSI status. While immunohistochemistry (IHC) and MSI testing are widely used, both methods can sometimes produce variable results. For instance, IHC can yield false negatives due to somatic mutations, complicating the evaluation of mismatch repair proteins. Upfront testing using both IHC and MSI-polymerase chain reaction could help overcome these limitations and improve patient selection for combination therapies.42Expand Reference
Exploring the combination’s efficacy in earlier lines of therapy is another promising avenue. By integrating TAS-102 earlier in the treatment regimen, researchers aim to improve long-term outcomes and potentially increase the survival rates for patients with mCRC. This strategy could redefine the treatment landscape for colorectal cancer, offering new hope for patients who have limited therapeutic options.
Conclusions
The journey of trifluridine/tipiracil (FTD/TPI) in managing refractory mCRC offers a glimmer of hope amidst challenging circumstances. The early phase I trials laid the groundwork, providing invaluable insights into dosing and safety parameters. Subsequent studies, such as BiTS, further affirmed the efficacy and tolerability of FTD/TPI, particularly in combination with BEV, inspiring ongoing investigation.
While the RECOURSE trial demonstrated the standalone efficacy of FTD/TPI in refractory mCRC, it is the SUNLIGHT trial that truly highlights the potential of combination therapy. The SUNLIGHT study, with its encouraging outcomes – especially the enhancements in OS and disease control – emphasizes the transformative impact of pairing FTD/TPI with BEV.
Looking ahead, these trials instil hope not only for patients but also for the entire medical community committed to refining dosing strategies, expanding patient eligibility and exploring innovative combinations.