Malignancies of the lung are common, and are the leading cause of cancer deaths worldwide.1Non-small cell lung carcinoma constitutes the majority of these lesions, with small cell lung carcinomas comprising the remainder (15%).1Non-small cell lung cancers are subdivided into three histological subtypes: adenocarcinoma, large-cell carcinoma, and squamous-cell carcinoma.1The subtypes are associated with various mutations, and thus demonstrate different metastatic potential.2Furthermore, the heterogeneous nature of these mutations in the primary tumor produces a population of cells that have undergone the steps necessary in the metastatic cascade, leading to disease progression.3
Metastatic spread occurs in approximately 50% of lung malignancies, with the most frequent destinations being the lymph nodes (46–85%), adrenal glands (36–64%), liver (48–58%), bone (21–41%), and brain (14–45%).3–6Spread of lung cancer to the gastrointestinal (GI) tract occurs through deposition of malignant cells in the subserosal layer of the bowel7through the spinal vein hematogenous route or retroperitoneal and mesenteric lymphatic routes,8with subsequent proliferation of these new foci.9However, omental and abdominal lymph node metastases suggest that direct extension into the GI tract can also constitute a significant portion of such metastatic lung cancer.10Overall, metastatic disease to the GI tract has always been believed to be exceedingly rare.11Interestingly, post-mortem studies of lung cancer patients have found a much higher incidence of GI metastasis, indicating that it must be predominantly clinically silent.
General features
The clinical presentation of metastatic lung carcinoma to the common sites is well known. Metastatic disease to the GI tract produces few, if any symptoms that are mostly of ambiguous nature, resulting in consideration of more common disease processes. In fact, the majority of reported cases occur with patients lacking abdominal symptoms and lesions are later revealed at autopsy.12
When present, symptoms may pertain to the specific location of the metastatic lesion. For instance, a secondary tumor in the small bowel may present with obstruction due to intussusception, GI bleeding, perforation of the bowel, or even fistulization.12–15 The most common symptom is abdominal pain, which is found to occur in only 50% of patients with metastasis to the GI tract.16 Less commonly encountered symptoms include obstruction, perforation, GI bleeding, dysphagia, anemia, jaundice, and bowel habit change.16Although some of these may not disclose an obvious cause, additional disorders such as iron deficiency anemia may develop as a result, thus increasing suspicion for less common etiologies.17 Reported cases with such presentation have
involved a precipitous drop in hemoglobin due to multiple small bowel lesions post-diagnosis of primary lung carcinoma18 or as a single solitary lesion in the colon resulting in a retrograde diagnosis of a previously occult lung cancer.17 Finally, as with any malignancy, constitutional signs lacking specificity may be present and include nausea, vomiting, and weight loss.12
The clinical outcome of GI metastasis from lung cancers has been variously described. Generally, it is expected that advanced disease is present when metastasis to the GI tract occurs, thus implying poor prognosis.7,19One analysis by McNeill et al. estimated survival of fewer than 16 weeks.12 Another review of the literature by Lou et al. had determined a similarly poor prognosis of colonic metastasis having an overall survival ranging from five weeks to one year, with a median of three months post-diagnosis.11The frequency of asymptomatic cases, lack of specificity for etiology of symptoms when present, and overwhelming spectrum of disorders that must be considered beforehand has made metastatic carcinoma of the lung with GI involvement relatively silent in the clinical setting. This contributes to the poor prognosis. However, with the emergence of modern chemotherapy and advanced care for lung cancer patients, a longer life expectancy may be predicted, and with that an increasing number of cases presenting with such metastatic disease.12
Diagnosis
Primary lung cancer that metastasizes to the GI tract usually presents as a solitary nodule, with preponderance for the male sex (80%).20Due to the lack of presenting symptoms, it can be an exceedingly difficult entity to diagnose, and as a result is often incidentally revealed. The nature of the symptoms makes a detailed history and physical examination with focus on possible extra-thoracic manifestations essential. Efficiently ruling out other etiologies of these manifestations and maintaining a high degree of suspicion can lead to an earlier diagnosis.
As part of the physical examination, fecal occult blood testing (FOBT) may detect bleeding from secondary GI lesions in much the same way it would be used as a screening test for colorectal carcinoma. As there is no literature concerning FOBT efficacy in detecting metastatic lung cancer to the GI tract, it is presumed that similar figures would be encountered with this clinical entity. Studies with regards to colorectal cancer have determined that FOBT is not a very sensitive test for uncovering advanced neoplasia in the GI tract. A study by Lieberman and Weiss had found a positive FOBT in only 23.9% of patients with advanced neoplasia of 10 mm or larger in size, with an overall sensitivity for any neoplasia determined to be 11.7%.21Many cancers present in the GI tract do not actively bleed, and may therefore bypass a positive FOBT result.22Thus, FOBTs have the capability of incidentally revealing a lesion, but can neither rule in nor rule out malignancy in this location.22 In addition, FOBTs are limited to detection of lesions causing lower-GI bleeding, and inadequately uncover lesions from the upper-GI tract.23Nakama and Zhang demonstrated that FOBT was inadequate for determination of gastric cancer, providing a positive test result in only 14.3% of patients with a positive predictive value of 1.1%.23 Furthermore, it was found that the rate of detection for gastric malignancy was 0.13% and 0.15% for negative and positive test results, respectively, thus exhibiting no significant difference.23 Although FOBTs lack sensitivity, they are less invasive and more cost-effective than other methods of screening for malignancies in the GI tract.22
An abdominal computed tomography (CT) scan is of critical value in detection of an intra-abdominal mass. The retrospective study conducted by Kim et al. used 31 patients with confirmed metastatic lung cancer to ascertain the sensitivity of CT scanning in detection of GI lesions. Of the 43 pathologically confirmed metastatic lesions in 28 patients, 31 were detected via CT scan.16This estimates the sensitivity via CT scanning at 72%. Most often, these findings demonstrated wall thickening (14 cases) or an intraluminal polypoid mass (14 cases).16Other radiographical signs were not as commonly exhibited in this study, such as non-bulky lymphadenopathy in the adjacent mesentery (25%) and small-volume ascites (21.4%).16Features that can also induce suspicion in the case of metastatic gastric cancer include volcano-like lesions or a mass with the ‘bull’s-eye’ sign of umbilication.19A disadvantage of this diagnostic tool is its limitation in detecting smaller masses. This is apparent through the predominance of esophageal metastases being microscopic in nature24 and in some case reports involving other organs of the GI tract. For instance, the secondary lesion in the transverse colon (<5 mm) was undetectable via CT scan in one case report of a primary lung adenocarcinoma later confirmed via endoscopic biopsy.17Interestingly, an approach has been used in some cases to detect a tumor ‘blush’ in association with CT mesenteric angiography and angiogenesis.18Evidently, CT scanning as a modality for simple detection of intra-abdominal metastasis of lung cancer is of great importance, but limited to the size of the secondary lesions.
Positron emission tomography (PET) scanning has been advocated as a useful diagnostic modality for detecting GI metastasis.12This modality has detected asymptomatic GI lesions from a primary lung cancer as described in literature,25and is advocated by Hayasaka et al., finding four GI metastases from primary lung tumors in 308 patients undergoing a whole-body PET scan.26However, the role of this modality is not clear due to the relatively few cases of GI metastasis from primary lung cancer and the general lack of such clinical data.12 Furthermore, the relative
insensitivity of this test is apparent through an estimated rate of 25% in the detection of extra-thoracic lesions of lung cancer.11,27Another imaging study that has been employed in the detection of GI metastasis reported in literature is Tc-99m red blood cell-labeled scintigraphy. This modality may be employed for cases of overt GI bleeding, able to detect bleeding rates as low as 0.05–0.1 mL/min.28
Endoscopy studies may be utilized when GI bleeding has raised suspicion for a metastatic lesion and less invasive studies have not determined causality. Esophagogastroduodenoscopy can have an overall accuracy of more than 90% in detecting upper-GI bleeding.28Many cases of metastasis to the esophagus have been similarly documented via upper endoscopy.7,24,29,30Its advantage is that biopsy may be taken for histologic confirmation of a secondary lesion.11This is necessary to determine optimal therapy to provide the best possible prognosis. Of note, squamous cell carcinoma of the lung is known to be the most difficult to treat, and is one of the most frequent causes of GI metastases.3Similarly, large-cell and small-cell carcinomas contribute to the most frequent GI metastases (Table 1). Immunohistochemical staining is essential for the clarification between malignancies of pulmonary and GI origin.19Stains for TTF-1, CK7, and CK20 are utilized to identify carcinomas of lung origin.5,17,31A positive TTF-1 stain is of importance in the case of lung adenocarcinomas, in which TTF-1 differentiates between an adenocarcinoma of lung from colorectal origin.32This occurs with a sensitivity of 57.5–76% and a specificity of 99–100%.19Additionally, positive staining for CK5/6 or p63 with negative staining for CK20 and CDX-2 typically represents adenocarcinoma of the lung; a positive stain for CDX-2 rules out adenocarcinoma from the lung.11 These determinations can be used to plan management and predict prognosis.
Thus, given the presumed rarity and elusive nature of metastatic lung cancer to the GI tract, there is no definitive diagnostic test to be utilized demonstrating specificity under such a degree of low suspicion. Often, when a GI metastasis is discovered, it is of incidental nature and this may be why it is much more commonly appreciated at autopsy when compared to the clinical setting.
Comparison of clinical cases with post-mortem studies
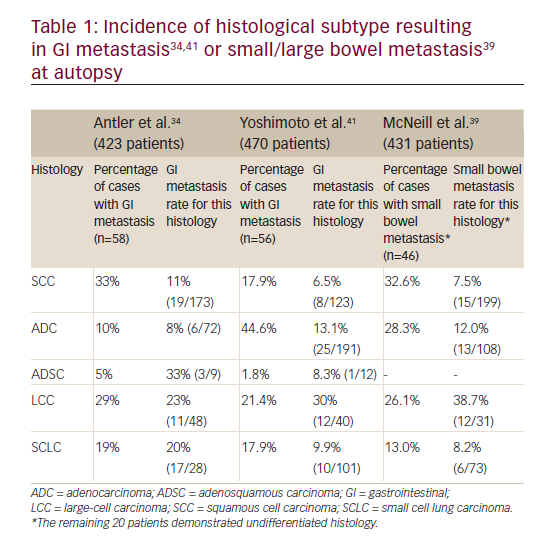
Various studies have demonstrated a discrepancy between the estimated incidence of GI metastases from a lung cancer in the clinical setting and at autopsy. Supporting this theory, many experts believe that up to 30% of patients undergoing resection for cure of lung cancer have silent metastatic disease.33 One factor attributable to the under-diagnosing of these secondary lesions may be the assumption that symptoms are related to side effects of chemotherapy.16,31 Considering squamous cell carcinoma and large-cell carcinoma are the most commonly appreciated histological subtypes involved in GI metastases (Table 1),34 suspicion for this type of disease progression should be emphasized for patients with newly diagnosed lung cancer of these types.
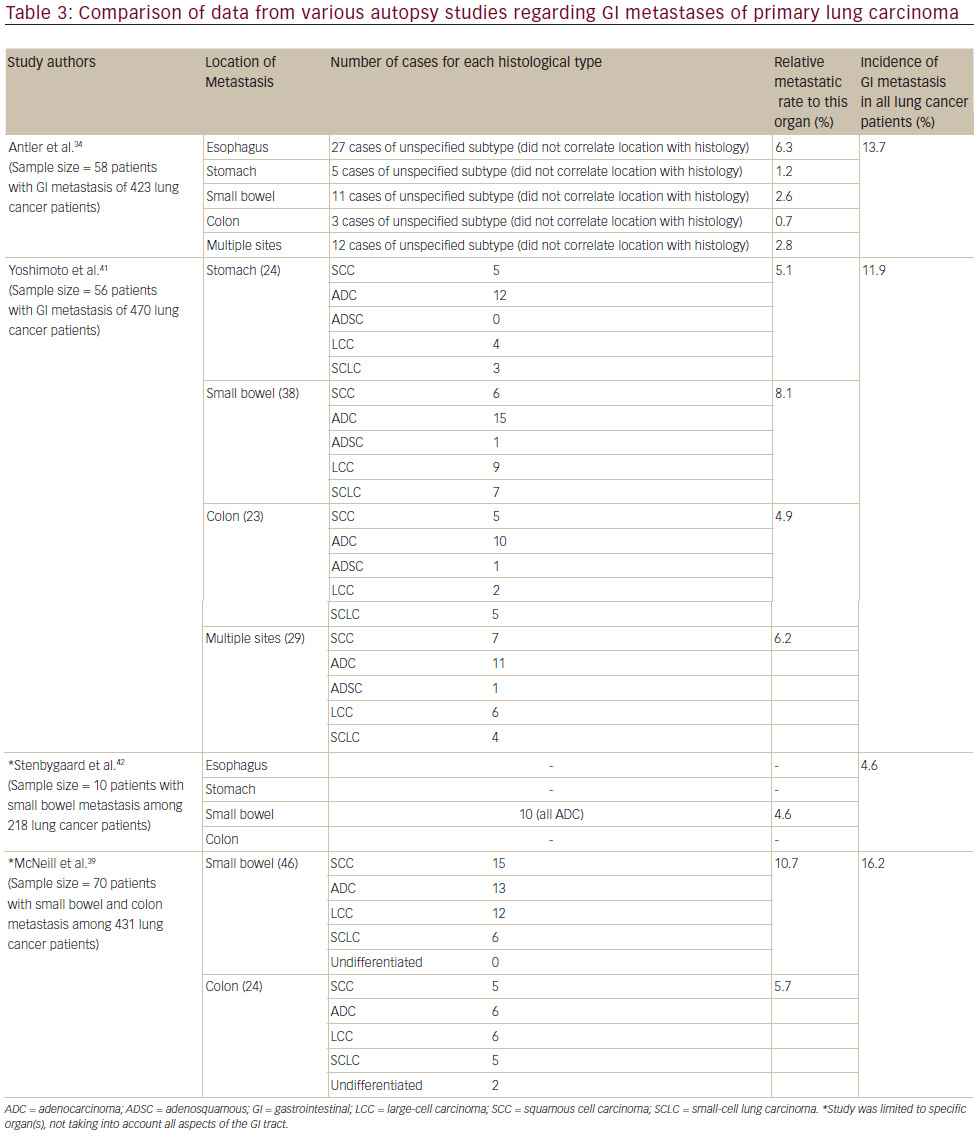
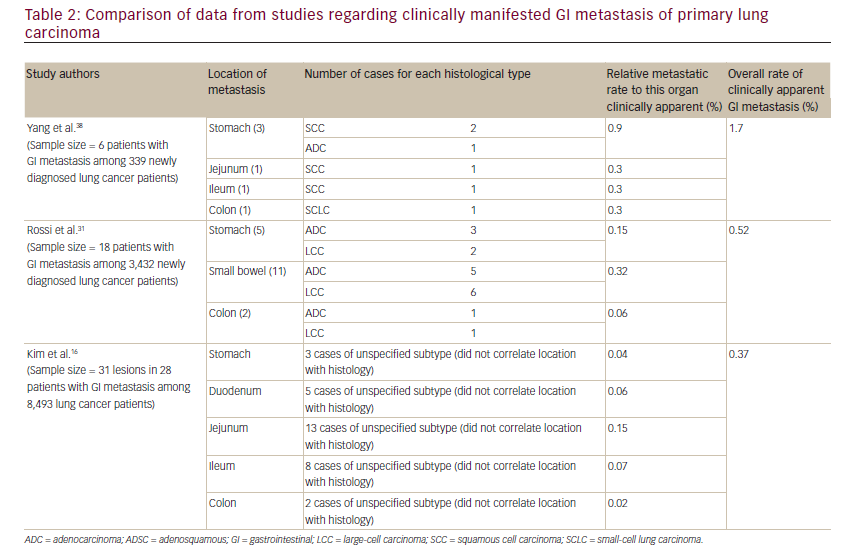
The total number of cases reported metastasizing to the stomach is less than 60,35to the small intestine it is 58 cases36and to the colon there are 14 that are apparent in the literature.4,11–13,17,27,37The estimation of symptomatic GI metastasis varies. A number of studies have estimated the incidence to range from 0.2–1.7% (see Table 2).11,16,18,38Evidently, such a disease entity is expected to be exceedingly rare. However, the studies investigating incidence at autopsy have demonstrated different results, producing some ambiguity as to the actual incidence. It has been estimated that there is a 4.6–14% incidence of GI metastasis found at autopsy (see Table 3).11,31,34,39Antler et al.,34Yoshimoto et al.,41 and Burbige et al.40demonstrated a metastatic rate of 14% (58 of 423 lung cancer patients), 11.9% (56 of 470 lung cancer patients), and 12% (18 of 147 lung cancer patients), respectively. The study by Stenbygaard et al. produced the lowest metastatic rate to the GI tract (4.6%).42This may due to the fact that this study was limited to the adenocarcinoma histology alone; it is known to be less aggressive than squamous cell carcinoma,3and constitutes a lower proportion of lung cancer patients demonstrating such metastatic disease and has shown a lower metastatic rate (see Table 1).Additionally, Stenbygaard’s postmortem study only quantified the number of metastatic lesions to the small bowel without evaluating other parts of the GI tract.
However, it is evident that there is a difference between incidence of clinical cases and post-mortem studies. This is apparent through the relative lack of published literature and case reports regarding this issue when compared to the number of cases described in autopsy studies.
Esophagus
Esophageal lesions are the most common, accounting for 41% of all metastatic lung cancer lesions to the GI tract.34 Unfortunately, to the best of our knowledge, supporting studies to confirm the predicted rate of clinically apparent esophageal involvement does not exist. The postmortem study by Antler et al. indicated that the actual incidence is 6.3% (see Table 3).19Mizobuchi et al. further support this in a retrospective autopsy study of metastatic lesions of the esophagus from any origin. In a sample size of 450 lung cancer patients,51patients demonstrated esophageal involvement postmortem, of which 34 lesions were only apparent on the microscopic examination.24This depicts a metastatic rate to the esophagus of 11.3%. An explanation as to why clinical incidence of metastasis to the esophagus is much less than that found at autopsy involves the rate of associated symptomology. In one study of lesions of primary lung cancer, only 15% of patients with esophageal metastases presented with dysphagia.43This may be associated with the fact that most of these lesions were only evident via microscopic examination, lacking the ability to produce anatomical symptoms.
Stomach
Clinical incidence of metastatic lung cancer to the stomach is considered extremely rare, presenting with symptoms in only 25% of cases,29and as a result is rarely diagnosed in living patients.45When present, these symptoms include upper abdominal pain (50%), anorexia (35%), anemia (27%), nausea and vomiting (23%), occult blood and melena (23%), and hematemesis (19%).44,45A possible explanation for why it is so clinically unapparent is the submucosal nature of most of these metastases to the stomach not being likely to develop symptoms.45The clinical incidence of gastric lesions from primary lung carcinoma ranges from 0.04–0.9% as extrapolated from retrospective studies conducted by Yang, Rossi, Kim, and their colleagues.
Autopsy studies by Antler et al. had estimated the incidence of metastatic lesions in the stomach from lung carcinoma to be 0.2% (see Table 3). Yashimoto et al. found the incidence to be 5.1% (24 of 470 lung cancer patients) in a similar post-mortem study.41 Evidently, the studies exhibit a relatively large range of which these secondary lesions can occur in the stomach.
Menuck, Oda, and their colleagues further illustrate the rarity of such metastatic lesions. They had performed post-mortem studies determining the frequency of metastatic lesions to the stomach of any origin. The overall metastatic rate to the stomach was 1.7% in 1,010 autopsy reports as per Menuck et al.40,44 Furthermore, of the 6,830 autopsy reports for gastric metastasis conducted by Oda et al., 85 (6.8%) had demonstrated lung cancer as a primary lesion.30
Small intestine
As with all of the GI lesions secondary to metastatic lung carcinoma, clinical presentation is rare due to the lack of symptoms. With localization to the small bowel, the most common manifestations are perforation (59%), obstruction (29%), and hemorrhage (10%).8Extrapolating from the data accumulated by Yang, Rossi, Kim, and their colleagues (see Table 2), it can be estimated that the clinically manifesting small bowel metastasis occurs with a rate of 0.6% (two cases of 339 lung cancer patients), 0.3% (11 cases of 3,432 lung cancer patients) and 0.3% (26 cases of 8,493 lung cancer patients), respectively.
This sharply contrasts with the conclusions made by autopsy studies. Yoshimoto had estimated the incidence of this disease state at 8.1% (38 cases of 470 lung cancer patients) (see Table 3). This is further supported by the post-mortem studies of Stenbygaard et al., Antler et al., and McNeill et al., estimating the metastatic rate to the small bowel at 4.6% (10 cases of 218 lung cancer patients), 2.6% (11 cases of 423 lung cancer patients), and 10.7% (46 cases of 431 lung cancer patients), respectively (see Table 3). Evidently, there is a significant difference between clinically manifested small bowel metastasis and the actual incidence.
Concerning the segments of the small intestine most often affected by such metastasis, the studies demonstrate contradiction. For instance, the autopsy study performed by Hillenbrand et al.36indicated that 55% occurred in the jejunum, 32% in the ileum, and 8% in the duodenum, with the remainder in multiple locations, while Antler et al. had shown that the ileum was by far the most common site for malignant cell deposition, with an equal predisposition for the duodenum and jejunum.34Of particular note, all the patients with small bowel metastasis had at least one other metastatic site outside of the GI tract,39indicating that GI metastasis occurs at a very late stage in pathogenesis of lung cancer.
Colon
Colonic metastasis seems to be particularly rare, with the reported cases few in number and varying in histological subtype.11Only a handful of clinically manifesting cases have been described in literature. In fact, to the best of our knowledge, only 14 such individual cases have been reported. This exhibits a considerable discrepancy between the clinical incidence and that of postmortem findings. Yang et al. estimated an overall rate of GI metastasis from lung cancer at 1.7%, with a specific colonic rate of approximately 0.5%.11,39,46 This is much higher when compared to the studies performed by Rossi et al. and Kim et al., which estimate the colonic rate as 0.06% and 0.02% as extrapolated from clinical data (Table 2).
Contrarily, autopsy studies have shown a much higher incidence of colonic metastasis, as exemplified by Yoshimoto and McNeill with their respective studies. Yoshimoto et al. estimated the actual incidence of this disease as 4.5% (21 colonic metastases of 470 lung cancer patients), and McNeill estimated this same rate as 5.7% (24 colonic metastases of 431 lung cancer patients) (see Table 3). Clearly, the incidence in clinically manifesting situations differs from the actual incidence of the disease.
Moreover, the predominant histology for colonic lesions was found to be large-cell or squamous cell carcinoma,15,34,46coinciding with incidence of other anatomical locations. Thus, it remains that newly diagnosed patients with these histologies should be under suspicion for clinically silent GI lesions.
Complications and management
GI metastasis of primary lung lesions can cause complications similar to other malignancies present in the GI tract, such as perforation, obstruction, intussusception, and GI hemorrhage.16However, studies have shown that small-bowel perforation occurs with metastatic lesions of lung cancer more frequently than with primary malignant tumors,47most likely due to the subserosal nature of such lesions. Additionally, perforation is predominantly associated with lesions of wall-thickening nature.16Bowel obstruction is another such complication, and has always occurred with intussusception,48with the secondary lesion acting as a leading point. The location that is particularly afflicted with this complication is the small bowel,34and commonly results in subsequent peritonitis.
Management is reliant on early detection of GI metastases for local management and staging of the primary disease. Physical capacity of the patient and prognosis of specific disease are factors in determining curative approach or palliation. This can involve ideal treatment via surgical resection of the GI lesion and subsequent chemotherapy.18 Chemotherapy for metastatic lung carcinoma is dependent on histology. Squamous cell carcinoma is known to be more aggressive with an associated poor prognosis.3This is supported by the fact that adenocarcinoma of the lung presents with fewer incidences of GI metastatic lesions in the study by Antler et al., at the same time as having more responsive treatment via erlotinib for cells mutated for epidermal growth factor receptor (EGFR) and crizotinib for cells mutated in anaplastic lymphoma kinase (ALK).3
One concern with treating GI metastases from lung cancers is the risk of chemotherapy-induced perforation.12 Yuen et al. have suggested that the increased necrosis from chemotherapy can elevate the risk of bowel perforation due to the subserosal nature of lesions and rapid division.49However, it is difficult to ascertain whether a perforation is due to an ulcerating mass or the chemotherapy itself.49This ambiguity as to perforation etiology is supported in the retrospective study by Kim et al. in which management with chemotherapy resulted in a 22.2% rate of perforation versus a 21.1% rate of perforation without chemotherapy.16
Surgical intervention is indicated in symptomatic cases; especially with emphasis on arising complications.18It is advocated that with complicated lesions such as those causing obstruction, bleeding or perforation, the secondary lesion should be managed with the appropriate surgery to palliate, increase quality of life and shorten hospitalization.13,18,32Logically, it can be inferred that resection of such a mass may also reduce the frequency of chemotherapy-induced complications such as perforation when treating the primary lesion.18 However, this mode of management is associated with considerable risks. The literature describes perioperative mortality ranging from 22–100%, with only one study reporting no perioperative mortality.18,39,47,50,51Thus, surgical management must be considered in a situational manner, assessing for the necessity of circumventing possible complications and the risk of perioperative morbidity or mortality.
Conclusions
There is an apparent discrepancy between symptomatic cases of metastatic lung cancer to the GI tract and post-mortem findings. Patients found to have ambiguous symptoms in the presence of a lung malignancy should be scrutinized with high suspicion for an evidently more common disease progression than expected. This should be emphasized with lung cancers of squamous cell and largecell histology due to their predilection for an associated aggressive presentation. This disease presentation may be encountered more often with the improvement of treatment options for lung cancers and novel approaches to identifying such cases must be investigated.













