Use of Anthracyclines in Acute Leukemia
Use of Anthracyclines in Acute Leukemia
Anthracyclines have been part of oncology treatment protocols since the 1980s, and antileukemic effects have been proved decades ago. Although anthracyclines are the keystone of leukemia treatment, there is no consensus for the optimal formulation, dose, or schedule in treating acute leukemia.1 Various anthracyclines have been evaluated in randomized clinical trials for their efficacy and toxicity. Due to the good antileukemic effect, most protocols contain a relatively high dose of anthracyclines. Unfortunately, anthracyclines have many side effects, of which cardiotoxicity (especially reduced ejection fraction and heart failure) is one of the most important. Cardiac muscle cells have limited regenerative capability, which may render the heart susceptible to permanent or transient adverse effects from chemotherapeutic agents such as anthracyclines. Toxicity ranges from treatable arrhythmias to potentially lethal conditions such as myocardial ischemia and cardiomyopathy. Endomyocardial biopsies in anthracycline-induced cardiomyopathy demonstrate sarcoplasmic reticulum dilation, vacuole formation, myofibrillar dropout, and necrosis.2,3 Cardiotoxicity can be a problem during treatment, but is especially known to occur in survivors of (childhood) cancer, as late effect.4–6 Acute cardiotoxicity is in fact a risk factor for the development of late cardiotoxicity, and one of the risk factors for acute toxicity is administration of anthracyclines by intravenous push, leading to high peak levels which are apparently cardiotoxic.7,8 Therefore, pediatricians always recommend intravenous infusion of anthracyclines for at least 1 hour.4 Cumulative dosages of anthracyclines above 300 mg/m² (depending on which review is employed) and high peak doses have been associated with clinically significant cardiotoxicity and are therefore widely debated, especially in pediatric oncology.3,4,7–10 Indeed, because of the development of the heart muscle, children are more vulnerable to drug-induced cardiotoxicity than adults.11
However, elderly patients can also be vulnerable because of pre-existing cardiac disease, and clinically relevant late cardiotoxicity has been reported in middle-aged adults treated with relatively high cumulative doses of anthracyclines as well.8
Anthracyclines, similar to many drugs used in the treatment of oncologic disease, also inflict damage to many other healthy cells. Accumulating the conventional drugs selectively in cancerous cells is difficult to achieve. Because of these toxicity concerns, dose limitations and reduced intensity of treatment is required. Besides this, many oncologic drugs have suboptimal pharmokinetics, biodistribution, and chemical stability. Therefore, recently, methods to improve these features have been studied, one of which are liposomes. In light of these developments, different liposomal formulations of anthracyclines became available.12–14
Liposomal Anthracyclines
Liposomes are closed vesicular structures consisting of one or more lipid bilayers. Because of their size and permeability, liposomes are easily altered and can be tailored to a variety of specific uses.
In vivo, conventional liposomal formulations are taken up primarily by phagocytic cells in the blood and by cells of the reticuloendothelial system (RES) in the liver, spleen, and bone marrow. Plasma proteins, immunoglobulins, and complement bind to the surface of the liposomes while they circulate. To avoid the effects on the RES, and of rapid uptake in the RES, specific formulations have been developed to avoid this uptake.12–14
Several biochemical, animal, and phase I studies have indicated that liposomal formulation of anthracyclines improve pharmacokinetics, reduce (cardio)toxicity, and have a better delivery capacity at the tumor site.12,13,15–19 Liposomal formulas, including liposomal daunorubicin (L-DNR), also tend to be effective against neoplastic cells, which are refractory to free anthracyclines.13,20 A 2012 meta-analysis on liposomal formulations of anthracyclines concluded: “The better therapeutic index of liposomal anthracyclines without compromising the efficacy makes it a favorable choice over conventional anthracyclines in elderly patients, patients with risk factors for cardiac disease and patients with prior use of anthracyclines.” Nevertheless, none of the available liposomal anthracyclines, compared with their free forms, has been proved to improve long-term survival directly.18
Liposomal Daunorubicin (DaunoXome®)
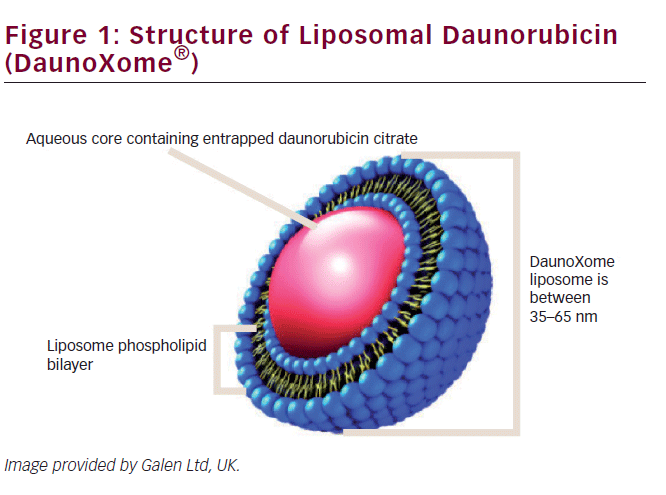
DNR is one of the most well-known anthracyclines and an important drug in many treatment protocols, in both adult and pediatric oncology. Unfortunately, as for all anthracyclines, cumulative high dose is the main risk factor for (irreversible) congestive heart failure.16,21,22 By creating a liposomal formulation of daunorubicin (see Figure 1), it was attempted to improve pharmokinetics and chemical stability of free daunorubicin, as well as to reduce toxicity.
L-DNR was one of the first liposomal formulated anticancer drugs. It consists of the citrate salt of daunorubicin, encapsulated within highly stable lipid vesicles (liposomes) comprising a bilayer membrane of distearoyl phosphatidylcholine and cholesterol. These remarkably stable vesicles prevent rapid and extensive uptake of the entrapped drug by the RES and its diffusion in most cells. L-DNR hereby demonstrates a lower clearance and a lower interindividual variability compared with free daunorubicin.12,23–25 In vitro studies have shown that L-DNR can increase tumor exposure up to 10-fold. Due to slow distribution of liposomes, about 100–200 fold greater area under the plasma concentration-time curve (AUC) has been reported.15,25,26 While in the circulation, the L-DNR formulation helps to protect the entrapped daunorubicin from chemical and enzymatic degradation, minimizes protein binding, and generally decreases uptake by normal (non-RES) tissues, such myocardial cells.17 L-DNR may also be (more) effective against multidrug-resistant leukemic cells.27
Data on the use of high doses L-DNR in patients with other malignancies reported little or no cardiotoxicity even when patients previously had been treated with anthracycline. These data support data obtained in adult patients treated for Kaposi sarcoma with high cumulative doses of L-DNR, even more than 1,000 mg/m2, in which hardly any cardiotoxicity was seen.28 In elderly patients L-DNR seems also less cardiotoxic compared with free daunorubicin, even at (very) high doses (up to 1,200 mg/m2).29
In summary, L-DNR, compared with free anthracycline, seems to be characterized by higher tumor cell delivery, improved pharmacokinetics, a better therapeutic index, and a more favorable toxicity profile. As monotherapy with L-DNR is not curative for any leukemia, we will focus this article on combination therapy with L-DNR.
Liposomal Daunorubicin in Acute Myeloid Leukemia
Standard acute myeloid leukemia (AML) induction therapy in children and adults usually contains three doses of an anthracycline, e.g. daunorubicin ≥60 mg/m2, idarubicin 10–12 mg/m2, or the anthracenedione mitoxantrone 10–12 mg/m2 and 7–10 days of cytarabine (arabinosyl cytosine/cytarabine [Ara-C], 100–200 mg/m2/day), whether or not combined with other agents, such as etoposide. With regimens combining an anthracycline with Ara-C, overall 80–90% of patients achieve complete remission (CR). When CR and survival rates improved, the interest in reducing toxicity grew and liposomal forms of antracyclines were studied.30
A meta-analysis reported a modest superiority of idarubicin compared with DNR at so-called equivalent doses in treating AML. When the DNR dose was intensified (applying a 5:1 conversion factor), the efficacy was similar.1
In 2005, Fassas and Anagnostopoulos reviewed the limited existing literature on the use of liposomal encapsulated daunorubicin in AML. There were no studies comparing free DNR with L-DNR in terms of efficacy and toxicity. Doses ranged from 120 to 300 mg/m2. All 12 of the described studies involved small phase I/II studies, most of them in patients with relapsed AML. Only one of them exclusively involved children.
Based on the results, the authors emphasized the usefulness of L-DNR. Treatment combined with Ara-C tended to be especially beneficial, as well as the use of L-DNR in relapsed or refractory AML. They concluded: “L-DNR can be used at high doses (up to 150 mg/m2) safely with acceptable toxicity. However, the superiority of L-DNR in terms of efficacy and toxicity will only be shown by prospective clinical studies comparing L-DNR with free daunorubicin or other regimens”.31
Adults
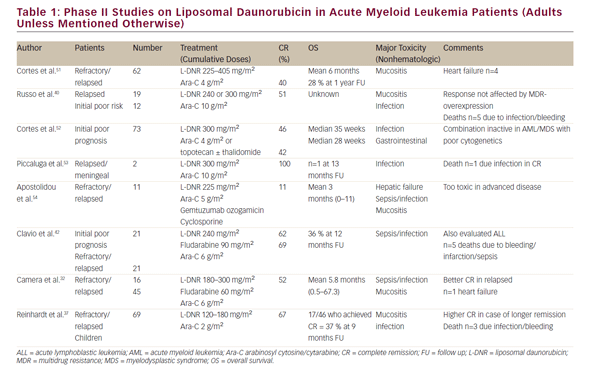
Several phase I/II studies (up to 2005) enrolling between two and 73 patients have been performed to evaluate the efficacy and toxicity of L-DNR in combination therapy (see Table 1). Overall conclusions were: L-DNR is well tolerated and effective, justifying further evaluation in treating relapsed/refractory or poor prognosis AML. CR rates varied from 40 to 60% (one study 100%, but comprising only two patients). One study reported an unacceptable toxicity with a regimen of L-DNR, Ara-C, and gemtuzumab ozogamicin, and cyclosporine, although it was unclear whether a particular drug caused this or the combination of drugs.31
Camera et al. treated 61 poor-risk AML-patients with the FLAD induction regimen (fludarabine, Ara-C and L-DNR 180–300 mg/m2). The overall CR rate was 52% (44 % and 56% for refractory [n=16] and relapsed [n= 45] patients, respectively]. A better CR rate (68%) was observed in the group of patients with a first CR duration >12 months (p=0.0002). Seven patients died during induction, mainly by sepsis. Twenty patients (62.5%) relapsed after first remission, with a median disease-free survival (DFS) of 7.3 months. Median overall survival (OS) was 5.8 months in the whole population, but 12.8 months in good responders. The authors concluded that FLAD proved to be effective, with acceptable toxicity in this group of patients. The response rate was better in the subgroup of relapsed patients compared with patients treated for refractory disease. Most of the surviving patients were those who proceeded to hematopoietic stem cell transplantation (HSCT), so the main indication for FLAD seems to be effective induction therapy in order to perform a transplant in remission.32
We only identified one randomized phase III trial comparing L-DNR and DNR in elderly patients with AML. In this trial, Groupo Italiano Malattie Ematologiche Maligne dell Ádulto (GIMEMA)s GSI 103 AMLE (2001–2004), free DNR plus Ara-C was compared with L-DNR plus Ara-C in 301 elderly patients (> 60 years of age) with AML. Eligible patients were randomized to receive one course of DNR (45 mg/m2 days 1–3) plus Ara-C (100 mg/ m2 days 1–7), or L-DNR (80 mg/m2 days 1–3) plus Ara-C (100 mg/m2 days 1–7). Among 153 patients in the DNR arm, 51% achieved CR, 35.9 % were resistant, and 13.1 % died during induction. Among 148 patients in the L-DNR arm, 49.3% achieved CR, 31.8% were resistant, and 18.9% died during induction. The L-DNR arm showed a statistically significantly higher incidence of early deaths (mainly by infection), but a lower incidence of relapse beyond 6 months. OS was slightly better in the L-DNR arm (borderline statistical significance [p=0.09]), but when accounting for risk profiles, the OS in the L-DNR arm was significantly better (p=0.003). The authors concluded that L-DNR seems to improve OS and event-free survival (EFS) with long-term follow up, because of a reduction in late relapses (relapse incidence at 24 months from 79% in the DNR arm to 59% in the L-DNR arm).33 Of note, elderly AML is a relatively chemoresistant disease, and thus not an ideal disease to demonstrate a benefit of a single drug.
Liposomal Daunorubicin in Newly Diagnosed Pediatric Acute Myeloid Leukemia
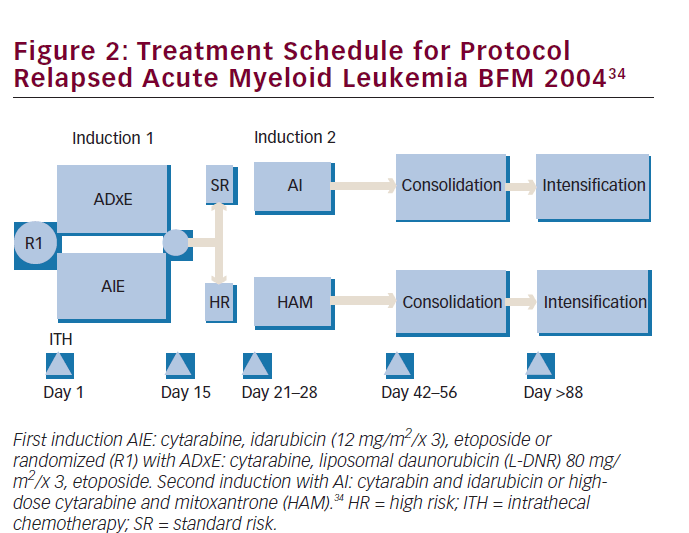
Two major randomized trials have been published in terms of regimens including L-DNR in childhood AML, and one concerned newly diagnosed patients. This multicenter, randomized clinical trial, AML-BMF 2004, enrolled 521 patients and compared a higher-than-DNR-equivalent dose of L-DNR at 80 mg/m2/dose for three doses with idarubicin at 12 mg/ m2/dose for three doses during induction therapy, always combined with standard therapy consisting of Ara-C and etoposide (see Figure 2). The investigators choose to dose increase liposomal DNR because they doubted, based on previous experience, that idarubicin could safely be increased—in contrast to L-DNR. This European L-DNR-study is the largest of its kind in pediatric AML. Overall probability of 5-year survival was not significantly different in both arms—L-DNR: 75% versus idarubicin: 76%. The same applied to EFS rates: 59% versus 53%, respectively. It should be noted that the study was underpowered to significantly discriminate small differences, a common problem in pediatrics due to relatively low patient numbers. However, L-DNR resulted in significantly lower treatment-related mortality and less toxicity, especially cardiotoxicity. The 5-year cumulative incidence of late and persistent (subclinical and clinical) cardiotoxicity in first CR (excluding those with transient cardiac dysfunction and those with either HSCT in first CR or relapse) were 0.7% in the L-DNR group and 1.8% in the idarubicin group. When subgroups were analyzed, t(8;21) AML patients had a significantly better EFS when treated with L-DNR. Explanation for this might be that t(8;21) patients have relatively slow blast cell clearance and thus benefit more from the prolonged effect of L-DNR. Overall the authors concluded that L-DNR at the given doses had an overall antileukemic activity comparable with idarubicin, with promising better activity in subgroups, and with less treatment-related toxicity.34
In Europe, L-DNR (three times 60 mg/m2/dose) was implemented in the Nordic Society of Pediatric Haematology and Oncology (NOPHO)-AML 2004 and Dutch-Belgian (DB)-AML-01 protocol in pediatric patients with >5% blasts at day 15 bone marrow puncture or, regardless of blast cell count, for patients with t(8;21) AML.35 Results have not been reported yet.
Liposomal Daunorubicin in Relapsed/Refractory Pediatric Acute Myeloid Leukemia
Although prognosis of childhood AML has improved over the last decades, OS rates are not higher than 70–75% at best.30,36 The high frequency of relapse, occurring in 30–40% of the patients, is one of the major problems. Probability of survival from relapse is low, but 5-year OS rates after first relapse AML have increased from 18 to 31% during the past two decades. This effect is most certainly because of uniform curative treatment concepts, including increased intensity of reinduction therapy, and because of improved supportive care regimens.36
Only one of the studies included in the review by Fassas et al.31 exclusively involved children (see Table 1). Reinhardt et al. described 69 relapsed AML patients, who received two induction courses with L-DNR at 60 mg/m2 and Ara-C. A total of 67% of patients achieved CR, especially those who had a long first remission period before relapsing.37
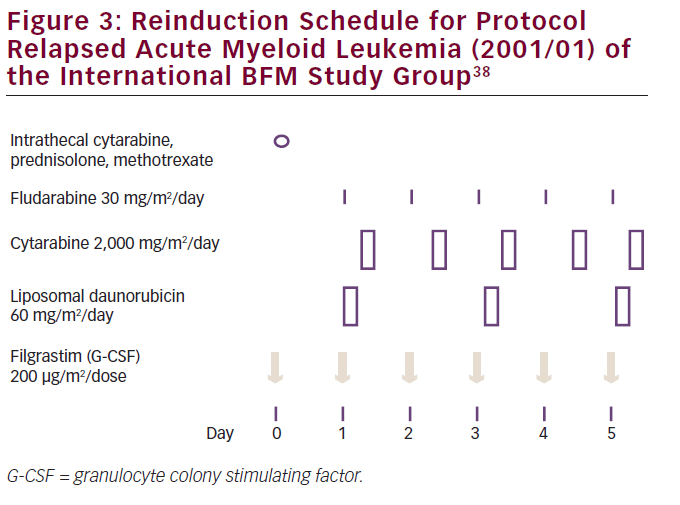
Due to a lack knowledge concerning optimal reinduction therapy in relapsed/refractory pediatric AML, a worldwide multicenter randomized trial was performed between 2001 and 2009 by the International BFM Study Group. The study compared reinduction therapy with more or less standard treatment with FLAG (fludarabine, Ara-C plus granulocyte colony stimulating factor [G-CSF]) to FLAG plus L-DNR in 394 patients (see Figure 3). The authors recently reported an improvement of 10% in early treatment response with L-DNR, based on the so-called day 28 bone marrow examination, which was the primary endpoint of the study. In addition, the CR rate was higher with L-DNR plus FLAG compared with FLAG only: 69% versus 59%, respectively. EFS and long-term OS were similar, but analysis of subgroups showed a significantly improved survival of core-binding factor-AML patients [with either t(8;21) or inv(16)] when treated with L-DNR: 82% versus 58%, respectively.
A possible explanation for the lack of improved OS with L-DNR may be due to insufficient consolidation treatment; moreover, the study was not powered to detect such an improved OS, again because of the lack of enough patients for this type of clinical study. Importantly, clinically relevant side effects including cardiotoxicity did not significantly increase in patients treated with FLAG plus L-DNR, although follow up is too short to draw conclusions on late cardiotoxicity.38
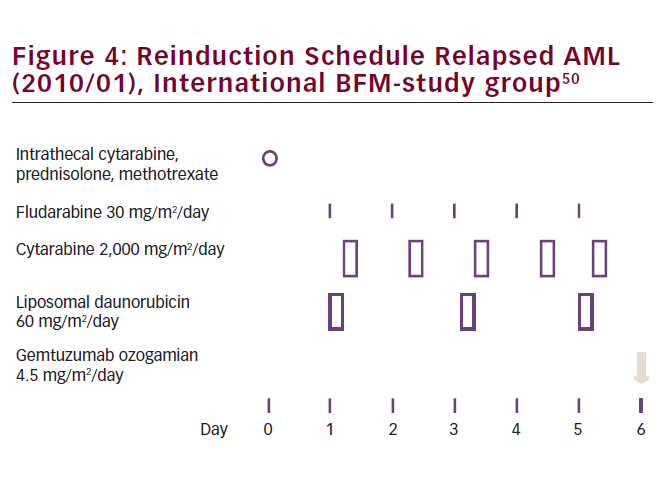
Currently, most protocols for reinduction therapy in pediatric AML are based on Ara-C plus an anthracycline. The available literature suggests that liposomal anthracyclines, such as L-DNR, are preferred as they are potentially less cardiotoxic, which is especially relevant in the setting of retreatment and previous exposure to anthracyclines. Future studies in pediatric relapsed AML will focus on improving induction and consolidation treatment, with novel agents such as gemtuzumab ozogamicin or bortezomib (see Figure 4), aiming at improved survival from relapse.38 Ultimately, relapses are ideally avoided or successfully treated at the stage of molecular disease already, such as is being carried out successfully in acute promyelocytic leukemia.
Liposomal Daunorubicin in Acute Lymphoblastic Leukemia
Anthracyclines are widely being used in the treatment of acute lymphoblastic leukemia (ALL). Although several studies demonstrate efficacy of anthracyclines, and there is evidence that increasing DNR doses improves outcome in poor prognosis ALL,39 few clinical studies on L-DNR in ALL have been performed. To the best of our knowledge, there are no comparative studies on DNR versus L-DNR in the treatment of ALL. We found six recent (2000–2010) phase II studies on L-DNR in ALL (see Table 2), but no prospective randomized clinical trials.
Russo et al. described efficacy of L-DNR combined with Ara-C in acute leukemia patients, both AML and ALL. Eleven patients with relapsed ALL were treated with L-DNR 240–300 mg/m2 plus Ara-C 10 g/m2. The CR was (unexpectedly) very high: 91%. Toxicity was mild and no cardiotoxicity was reported. The authors concluded that L-DNR can be of great importance in the treatment of ALL, but that the high CR rate in the small patient numbers needed confirmation in larger prospective trials.40
Nonpublished data (abstract only) by Krishnan et al. included 17 relapsed/ refractory patients (10 ALL, seven non-Hodgkin lymphoma) into a singlearm treatment with FLAG-X: fludarabine, Ara-C, G-CSF, L-DNR (240 mg/ m2). Eleven patients achieved CR and one patient partially responded. Nonhematologic toxicity was modest, but long-term follow up of cardiotoxicity was still ongoing. Eleven of the 12 responders proceeded to a transplant procedure. One died after FLAG consolidation before transplantation could be performed. After transplantation, five patients subsequently relapsed and four patients died from infection. The authors concluded that the FLAG-X regimen is a feasible and safe option for patients with relapsed or refractory ALL and, in general, high-risk lymphoid malignancies. However, long-term survival rates suggest that this group requires more intensive consolidation prior to transplantation.41
Clavio et al. described 62 patients with high-risk leukemia, of which 17 patients had relapsed/refractory ALL. All patients were treated with FLAD: fludarabine, Ara-C, and L-DNR (240 mg/m2). They had previously been treated with a somewhat similar regime including vincristine, cyclophosphamide, an anthracycline ± Ara-C. FLAD was well tolerated by most patients and nonhematologic toxicity was mild. Main problems involved infection and sepsis. Five patients died before response evaluation (one of infection, one of intestinal infarction, one of acute myocardial infarction, and two of cerebral hemorrhage). Among ALL patients, 10 achieved CR (59%). Six patients relapsed, of which four patients died of disease after relapse. In this subgroup of patients median duration of DFS and OS was 8 months (range: 2–14) and 9 months (range: 2–15), respectively. Especially poor prognosis karyotype [complex karyotype or t(9;22)] seemed to benefit FLAD management, since five out of seven such patients achieved CR (71%). The authors concluded that the FLAD regimen is a promising treatment in ALL patients who fail initial treatment, especially those with poor prognosis karyotype. Patient numbers, however, were small and the promising results need to be confirmed by larger and preferably randomized trials.42 Clavio et al. referred to a study by Baccarani et al. (abstract only), in which a very high CR rate (91%) was obtained by combining L-DNR and Ara-C in a series of 11 relapsed ALL patients. Major complications were infection and mucositis. One patients developed atrial fibrillation.42,43 Unfortunately, we were not able to obtain a full article on these patients.
Candoni et al. studied the efficacy and toxicity of reinduction therapy with L-DNR 240–300 mg/m2 plus Ara-C 10 g/m2 in 25 relapsed ALL patients. They also analyzed multidrug resistance (MDR)-related proteins. Twenty patients (80%) achieved CR, despite expression of one or more MDR proteins. Toxicity was mild, without any cardiotoxicity. OS was 39% after 12 months. The authors concluded that L-DNR has a promising role in ALL treatment because of the high response rate, without increased toxicity and despite overexpression of MDR-related proteins.44
In an attempt to improve treatment of de novo ALL or lymphoblastic lymphoma by intensifying anthracyclines during consolidation, Thomas et al. studied standard hyper-cyclophosphamide, vincristine, doxorubicin, dexamethasone (CVA D)-regimen (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) in combination with L-DNR and rituximab (n=68). Sixty-three (93%) patients achieved CR. DFS (follow up 90 months) and OS were 46% and 44%, respectively. Compared with 208 patients with standard hyper-CVA D treatment, there were no significant differences (DFS 50% and OS 53%). They concluded that early anthracycline intensification with L-DNR did not improve outcome for adults with de novo ALL or lymphoblastic lymphoma.45
Elderly
Offidani et al. enrolled 17 elderly patients (≥ 60 years) in a prospective study with an induction phase based on VDXD: vincristine (four doses 2 mg/m2), L-DNR (six doses 50 mg/m2), and dexamethasone (8 days 40 mg/m2). In 2003 they published the results of the first 15 patients.
Eleven patients (73%) achieved a CR, one (7%) had resistant disease, and three patients (20%) died during induction phase. Median DFS and OS were 21 months and 23 months, respectively. Two-year DFS was 36% with a 2-year OS of 38 %.46
In 2007, the authors published data on 17 patients treated with VDXD compared with 17 patients treated by standard GIMEMA ALL 0288 regimen: first induction with vincristine (four doses 2 mg/m2), DNR (four doses 40 mg/m2), prednisolone (14 days 60 mg/m2 plus 14 days 40 mg/ m2), and L-asparaginase (10 doses 6,000 IU/m2). Three patients died during VDXD-induction (compared with six treated with the 0288 regimen, a significant difference). One patient receiving VDXD had resistant disease compared with four in the 0288 arm. CR was reached by seven (41%) in 0288 patients versus 13 (76.5%) in VDXD. The most important nonhematologic side effects were infection and bleeding. Two patients treated with 0288 developed cardiotoxicity, and one did in the VDXD arm. Median EFS was 3.9 and 12.8 months, respectively (p=0.05). Median OS was 4.5 and 21 months (p=0.24) respectively. The authors concluded that the VDXD regimen, based on high doses L-DNR, seemed to improve outcome in elderly patients. The prognosis, however, remained poor.47
Children
We only found one report concerning L-DNR in childhood ALL. Sedki et al. evaluated, on a compassionate use basis, the combination of L-DNR and PEG-asparaginase as a salvage treatment in refractory/relapsed childhood ALL. Nine patients received 300 mg/m2 L-DNR and 2,500 IU/m2 PEG-asparaginase combined with vincristine and a corticosteroid. Eight out
of nine patients (89%) achieved CR. Major toxicity consisted of infections and mucositis and one patient experienced grade I cardiac dysfunction (at a cumulative dose of anthracyclines of 446 mg/m2). All eight patients were subjected to transplantation, and four children died from transplantrelated disease. Two children relapsed post-transplantation and died of disease progression. OS at 4 years was 22% (n=2). The patient with grade I cardiac dysfunction responded to an angiotensin converting enzyme inhibitor and presented a favorable outcome at 1-year post transplant, with normalization to its initial cardiac shortening fraction.48
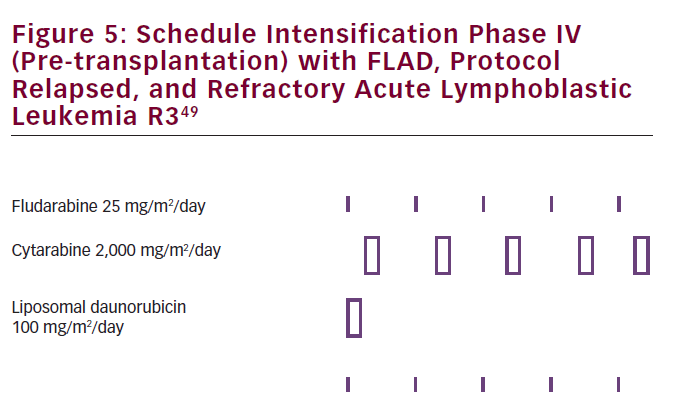
The authors concluded that this regimen can be used as a salvage treatment after failing at least two antileukemic treatments, allowing children to proceed to transplantation. This should, however, be further evaluated in a phase II trial. Overall prognosis of relapsed ALL in children remained poor.48 L-DNR has already been implemented in pre-transplant cytoreduction regimen with FLAD (see Figure 5) in pediatric relapsed/ refractory ALL.49
Conclusion
Preclinical studies, several phase I/II studies, and (a limited number of) three randomized clinical trials demonstrated improved pharmokinetics and chemical stability of L-DNR, with at least similar efficacy and significantly less toxicity compared with daunorubicin in its free form, in acute leukemias. Because of the liposomal formulation, higher doses of L-DNR can be applied, with equal or even less toxicity, providing improved outcome in several studies in the total population or in subgroups of patients. At this moment there is no solid evidence that L-DNR improves OS in de novo and/or relapsed acute leukemia, but it does improve EFS in core-binding factor AML patients in both settings. The use of L-DNR in pediatric patients is stimulated by the fear for late cardiotoxicity on the one hand, and the indications (although no final proof yet) that L-DNR is less cardiotoxic than free anthracyclines. L-DNR is already being implemented in several pediatric treatment protocols for relapsed AML patients50 and patients with t(8;21) AML or a poor initial treatment response.35 In adults, avoiding cardiotoxicity is important as well, and the data suggesting that higher doses of L-DNR can be used without increased toxicity is of special interest in view of recent studies that demonstrated improved outcome in adult AML patients with escalating doses of anthracyclines.
The role of L-DNR in ALL patients seems promising in those with poor risk, relapsed/refractory ALL, apparently leading to higher CR-rates and OS compared with standard regimen. Nevertheless, prognosis in refractory/ relapsed ALL remains poor and identifying new therapeutic targets remains necessary, as well as optimization of treatment schedules with conventional agents, including L-DNR.