The past decade has experienced a shift in the treatment landscape for multiple myeloma (MM), with the introduction of novel agents and new combination therapies which have improved survival outcomes for patients across all disease settings.1 These advances in treatment strategy have extended median relative survival to 7.7 years in patients with myeloma aged 65 years or younger and to 3.4 years in those aged 66 years and older.2 The most recent therapeutic approaches in the relapsed/refractory MM (RRMM) arena include the new immunomodulatory drug (IMiD) pomalidomide; proteasome inhibitors ixazomib and carfilzomib; and the introduction of immunotherapy, including the therapeutic monoclonal antibodies (mAbs) daratumumab and elotuzumab.
This review will examine the key treatment challenges and unmet needs which currently exist in RRMM including the adverse impact of treatment on quality of life (QoL), the toxicity burden of therapy and issues of clonal evolution and treatment-resistant disease. We will look at how these issues create particular challenges in the management of certain populations of patients with MM, including elderly or unfit patients, patients with high-risk cytogenetic abnormalities and those with toxicity-related complications and comorbidities. By examining the available clinical efficacy and safety data for the novel approved agents in RRMM in these specific subgroups, we aim to show how treatment can be tailored and targeted to meet individual patient needs and thereby provide optimised clinical care for RRMM.
In the relapsed setting, optimal management of MM is complex and European Society for Medical Oncology (ESMO) guidelines indicate that the selection of therapy should be guided by a number of different parameters including: patient age; performance status; comorbidities; the type, efficacy and tolerance of the previous treatment; the number of prior treatment lines; the available remaining treatment options; the interval since the last therapy; and the type of relapse.3 Relapses in MM may be clinical or biochemical, and in the case of biochemical relapse, salvage treatment can be delayed.3 For the youngest, fittest patients who have initially benefited from their first autologous stem cell transplant (ASCT), a second ASCT may be considered, although, this option is still infrequently used.3,4 Until recently, the benefits of salvage ASCT were unclear but recent prospective studies have shown that a second ASCT improves progression-free survival (PFS) and overall survival (OS) compared to conventional salvage therapy.4 In multiple, retrospective studies, salvage ASCT demonstrated efficacy after re-induction therapy in patients who relapsed after prior ASCT, with the duration of remission achieved after initial ASCT proving the key predictive factor for PFS.5–7
For most patients, the treatment approach will need to be based on prior exposure and toxicity.1 Wherever prior treatment was IMiD-based, current guidelines advise a switch to a proteasome inhibitor doublet (bortezomib or carfilzomib + dexamethasone) or bortezomib-based triplet therapy with dexamethasone and either daratumumab, panobinostat, elotuzumab or cyclophosphamide (Figure 1).3 Whenever the patient experiences first relapse after bortezomib-based induction, treatment should be changed to an IMiD-based treatment regimen with or without a novel agent. Other options include doublet lenalidomide, low-dose dexamethasone (Rd) therapy or triplets on an Rd backbone – for example, with the addition of daratumumab, carfilzomib, ixazomib or elotuzumab (Figure 1). If both IMiDs and proteasome inhibitors have been exhausted and the patient is experiencing a second or subsequent relapse, current ESMO guidelines recommend the alternative option of a clinical trial or daratumumab monotherapy if this has not been previously tried, while combinations based on a pomalidomide backbone with ixazomib, cyclophosphamide, bortezomib, daratumumab or elotuzumab (note: not all these triplet combination are currently European Medicines Agency [EMA]-approved) should also be evaluated.3

Current practice in relapsed and refractory disease
With the advent of novel agents, the therapeutic armamentarium for RRMM now contains numerous effective regimens; doublet or triplet regimens are preferred to single agents in order to achieve optimal outcomes, and thus are endorsed as best practice at first relapse. Compelling data from randomised, controlled phase III trials support the ability of novel agent-based triplets to achieve both superior response rates and prolonged disease control versus doublet combinations.8–14 Crucially, and in contrast to older triplet combinations, this superior efficacy can often be achieved with manageable additional toxicity.15 The clinical evidence is more robust for triplet therapy where novel agents are added to an Rd backbone; however, data also exist to support the role of bortezomib, low-dose dexamethasone (Vd)-based triplet therapy.16 It is beyond the scope of this review to discuss the underlying trial data in depth; however, ixazomib, carfilzomib, elotuzumab and daratumumab have all demonstrated statistically significant improvement in the primary clinical endpoint of PFS when combined with Rd versus Rd alone in patients with RRMM in several phase III studies.8,9,11,12 Significant improvements in PFS were also obtained with daratumumab or panobinostat when added to a Vd backbone compared to Vd in the relapsed/refractory setting in phase III studies.10,17 However, the clinical benefit of triplets may be less evident in elderly or frail patients. The choice of triplet constituents is crucial in these cases as some older or more unfit patients with poor performance status may benefit from less-intensive triplet regimens or dose reductions according to expert-based local guidelines.18–20
Despite its superior efficacy, not all patients will be able to tolerate triplet therapy and the cost of three-drug regimens may also prove prohibitive in some regions of the world.21 A recent retrospective cohort study examined factors associated with the use of triplets as second-line therapy in routine practice for RRMM management. This study showed that the majority of patients with RRMM did not receive triplet regimens; the rate of triplet use in clinical practice was only 23% in the time period between 2008 and 2015. Factors associated with triplet use in multivariate analysis were aggressive relapse, CRAB symptoms (calcium [elevated], renal failure, anaemia and bone lesions) and administration of prior triplet therapy. Patients treated with triplets also tended to be younger.22
Although multidrug regimens allow several disease pathways and more MM subclones to be targeted, it is important to consider the possible repercussions of triplet regimens in subsequent selection of active drugs in later lines of therapy. In particular the exhaustion of established options and drug classes, as well as the potential promotion of multiple drug resistance, may actually reduce the life expectancy of patients. Assuming that no overt toxicity arises, it is currently recommended that most regimens employed in the relapsed setting are continued until disease progression, in the same manner the clinical trials have been performed.23 However, prolonged therapy with current triplet regimens, particularly those incorporating the proteasome inhibitors bortezomib, carfilzomib or alkylating agents, can be difficult to employ in everyday practice due to the side effects. Barriers to sustainable long-term therapy include treatment-related toxicities such as peripheral neuropathy, fatigue, gastrointestinal symptoms, cardiovascular side effects, myelosuppression and renal complications, which are compounded by the requirements for regular clinic admissions and repeated injections or infusions. With some triplet regimens for RRMM, it appears reasonable to halt treatment once a stable plateau has been reached in order to minimise the risks of ongoing serious toxicity.24 It is also common for patients themselves to request a treatment-free period in order to reduce the need for clinic visits and alleviate drug-associated toxicities. This clinical picture may change in the future with the emergence of better tolerated triplet regimens and the possibility of triple-oral combinations.
Key treatment challenges in relapsed, refractory multiple myeloma
As MM progresses, mutations and genetic abnormalities accumulate, the malignant clone evolves and tumour behaviour becomes increasingly aggressive.25,5 As a result, the disease is rendered increasingly resistant to therapeutic control, responses are curtailed and periods of remission become shorter with each subsequent therapy.5 Prognosis is particularly bleak for patients who become refractory to both bortezomib and IMiDs.26,27 The management of RRMM presents particular challenges for clinicians and efforts to prolong survival must also be balanced carefully against preservation of QoL and minimisation of time spent in hospital, particularly in elderly and/or frail patients where a low-intensity regimen with a more favourable toxicity profile may be preferred.28
MM disease symptoms, medication-related side effects and suboptimal response to treatment can negatively affect patients’ health-related QoL (HrQoL).1 Evidence indicates that this effect is particularly pronounced in patients with advanced MM who typically carry a cumulative toxicity burden from several rounds of intensive treatment.28 With the uptake of new and increasingly complex drug combinations, the convenience and timing of dosing is another factor that can influence patients’ feelings about their prescribed therapy and their adherence to recommended regimens. Convenience encompasses several elements including the administration route (oral versus intravenous) of antimyeloma drugs and the need for dosing in a hospital versus home setting, which can prove taxing for patients and their caregivers.29
Treatment with bortezomib is associated with nervous system toxicity. Treatment-related peripheral neuropathy has also been reported to be almost 40%, although subcutaneous dosing is associated with decreased peripheral neuropathy.30 This risk is heightened in heavily pre-treated patients, who are more likely to have baseline peripheral nerve damage and this can often prove dose limiting.1 Thalidomide, no longer widely used in the US but retaining a key role in MM therapy in several regions of the world, is also associated with a high frequency of peripheral neuropathy and thromboembolic adverse events (AEs) in the RRMM setting.31–33 Myelosuppression is a recognised consequence of most established anti-myeloma regimens, with neutropenia, thrombocytopenia and anaemia ranking amongst the most frequently reported AEs.31 In addition, several standard-of-care antimyeloma medications require dose adjustments and reductions to allow safe dosing in patients with renal or hepatic impairment.31
Meeting unmet needs
Novel agents for MM provide the opportunity to address some of the major unmet needs in MM and overcome key limitations in existing therapy approaches. Evidence indicates that the improved clinical responses and prolonged progression-free intervals achieved with novel agents may translate into direct HrQoL benefits for patients. A clear link exists between positive clinical outcomes in MM and better HrQoL.1 In clinical trials, ixazomib maintained patient-reported QoL when added to Rd in RRMM patients, and additionally showed improved scores in the physical, emotional and social scales.34 Significantly better HrQoL was also seen in pomalidomide- and carfilzomib-treated patients in clinical trials when compared to standard comparator regimens that did not contain a novel agent.8,35 In general, newer compounds in the IMiD and proteasome-inhibitor class provide improved tolerability profiles compared with their predecessors and also offer specific advantages such as oral dosing options for previously intravenous-only drug classes.
Tailoring treatment to specific populations of patients with multiple myeloma
As well as overcoming some of the major limitations with existing therapeutic interventions for MM, the unique benefits and features of novel agents can help secure a ‘best fit’ treatment approach where therapy is tailored to meet individual patient needs (Table 1). 8–13,17,36–42
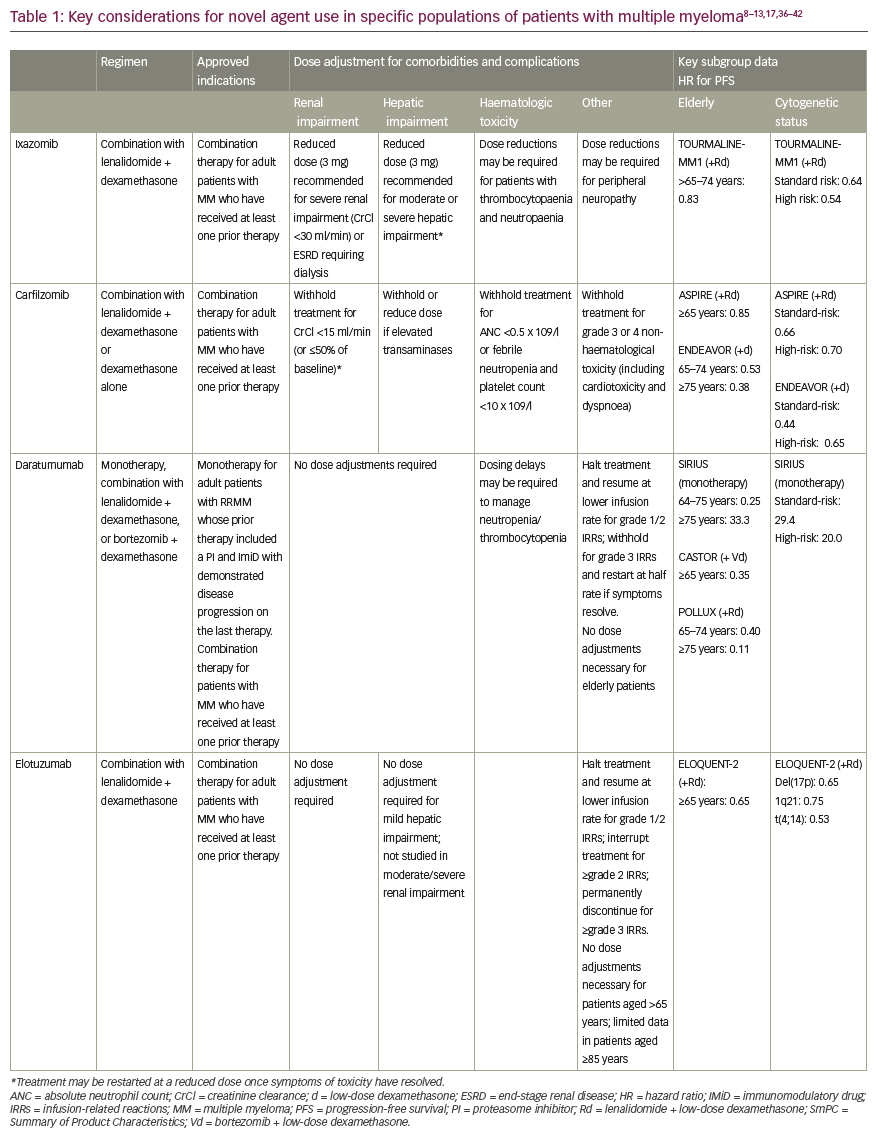
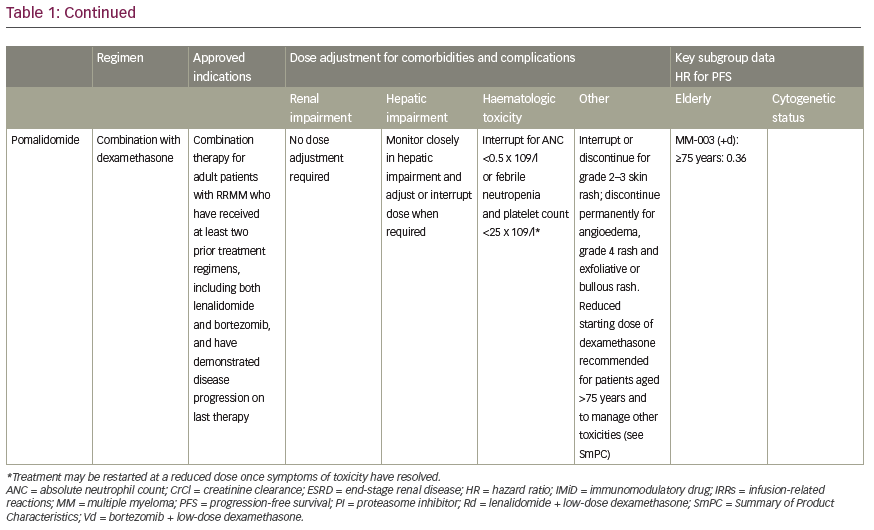
Complications of previous therapies and comorbidities
Neuropathy
In patients with risk factors for, or a history of, peripheral neuropathy, both ixazomib and carfilzomib are associated with lower rates of treatment-emergent peripheral neuropathy compared to bortezomib, making them particularly useful in this cohort of patients.7 In pivotal phase III studies in combination with Rd, peripheral neuropathy was reported in 27% of patients treated with ixazomib versus 22% in the placebo group (with grade 3 events occurring in 2% of patients in each group) and in 17.1% of patients treated with carfilzomib versus 17.0% in the control group.8,9 In its licensed combination with low-dose dexamethasone, pomalidomide had a peripheral neuropathy incidence of 15% (1% ≥grade 3) in the MM-003 phase III trial, while deep vein thrombosis and pulmonary embolism occurred infrequently with thromboprophylaxis.10 As both carfilzomib and pomalidomide have further demonstrated efficacy in individuals with baseline peripheral neuropathy, retaining a low rate of treatment-related neurotoxicity, these agents may prove particularly useful in this group of patients.8,43,44 Peripheral neuropathy has also been noted with daratumumab but only when this mAb is used in combination regimens with bortezomib.36
Renal impairment
Renal impairment is a frequent complication in MM, with approximately one in every five patients showing signs of kidney dysfunction as indicated by a creatinine level greater than 2 mg/dl.45 Bortezomib-based regimens have traditionally been the lynchpin of management of renal failure.46 New agents have helped to further expand treatment options for patients with renal impairment as no dose adjustment is required for either carfilzomib, panobinostat, elotuzumab or daratumumab in cases of reduced kidney function.31 In a 60-patient analysis, carfilzomib was commonly associated with a transient reduction of estimated glomerular filtration rate (eGFR) but improved renal function in 55% of patients with baseline eGFR of <60 ml/min/1.73 m2.47 Ixazomib too can be used at the full therapeutic dose in mild to moderate chronic kidney disease, although, dose reduction to 3 mg weekly is advised in patients with creatinine clearance of <30 mg/min.37 In contrast, dose reductions to 10 mg daily are indicated for lenalidomide in patients with moderate renal impairment and the recommended dose must be cut even further for those with severe renal impairment (7.5 mg daily or 15 mg every other day) or end-stage renal disease (5 mg/day).29 No dose adjustment is required for the IMiD pomalidomide in patients with renal impairment.38
Infusion reactions
The mAb therapies elotuzumab and daratumumab are hampered by none of the traditional peripheral neuropathy or thromboembolic toxicity issues associated with IMiDs or proteasome inhibitors, although myelosuppression remains a key side effect.36,39 However, infusion-related reactions are a new class of AE that clinicians must contend with when administering mAbs, and these appear at a markedly higher rate with daratumumab than with elotuzumab.29 In clinical practice, premedication and infusion adaption protocols must be employed to manage infusion-related reactions.36,39 For example, the rate of infusion-related reactions with elotuzumab was cut from over 50% to around 10% with the use of premedication measures.29
Cardiotoxicities
Cardiotoxicities have been documented with both IMiDs and proteasome inhibitors; however, carfilzomib in particular has been linked to a higher risk of cardiotoxic effects, especially hypertension, when used to treat MM – an important consideration for treatment selection in elderly patients with frequent cardiac comorbidities.31 In a detailed analysis of cardiac and renal complications in 60 consecutive patients with myeloma treated with carfilzomib-based regimens, 12% experienced a reversible reduction of left ventricular ejection fraction by ≥20%, an objective measure of cardiac dysfunction.47 Pooled safety analysis of four phase II monotherapy studies of carfilzomib showed an overall cardiac-related AE rate of any grade of 22%, with dyspnoea, hypertension and heart failure being particularly common.48 In an extensive literature review and meta-analysis of 29 carfilzomib trials, the overall estimated cumulative incidence of cardiotoxicity was 8.68% and 4.92%, respectively, for all-grade and high-grade (≥grade 3) toxicity – higher than for other proteasome inhibitors. Compared to the control group, the odds of developing cardiotoxicity due to carfilzomib was significantly higher with odds ratio of 2.03 and 2.04 for all grades and high grades, respectively. Concomitant immunomodulatory agents seem to increase the risk.49 This highlights the need for increased vigilance for cardiac AEs, including blood pressure measurements at each infusion and further consideration of optimal patient selection and risk mitigation strategies when treating patients with carfilzomib.
Infections
Infectious complications are responsible for important morbidity and mortality in MM, especially during the first 3–4 months of therapy. A differential impact of myeloma therapies on infection risk has been established, with the treatment phase also having an effect. In the RRMM setting, IMiD-based therapy was found to be associated with a severe infection rate of 16.6% in a recent meta-analysis.50 Therapeutic mAbs have also been implicated in increased rates of infection.11,36 In the ELOQUENT-2 trial, the incidence of infections, including pneumonia, was higher with elotuzumab treatment than in the control arm. Infections were reported in 81% of patients in the elotuzumab + Rd arm compared to 74% in the Rd group.11 Similarly, infection is listed as a very common adverse reaction with daratumumab therapy. Grade 3 or 4 infections have been reported with daratumumab-based combinations and background therapies (daratumumab, bortezomib, low-dose dexamethasone [DVd]: 21%; Vd: 19%; daratumumab, lenalidomide, low-dose dexamethasone [DRd]: 27%; Rd: 23%; and daratumumab, pomalidomide, low-dose dexamethasone [DPd]: 28%). Pneumonia was the most commonly reported severe (grade 3 or 4) infection across studies.36
The TEAMM (Tackling EArly Morbidity and Mortality in Myeloma) phase III study was a randomised, double-blind, placebo-controlled multicentre trial which evaluated the benefits of antibiotic prophylaxis on healthcare associated infections in 977 patients with multiple myeloma. Levofloxacin prophylaxis had a significant benefit in myeloma patients; febrile episodes and deaths were reported in 19% of patients who received levofloxacin within the first 12 weeks following diagnosis, compared to 27% of patients receiving a placebo drug. There was also a significant reduction in the number of deaths at 12 weeks with 8 patients in the levofloxacin arm versus 22 in the placebo arm.51
Elderly patients
Elderly patients pose complex management challenges in the RRMM setting. They are more susceptible to drug-induced AEs, less resilient to treatment-related toxicities and more likely to suffer with coexisting comorbidities.17 Polypharmacy is also a potential confounding factor, introducing the real risk of drug–drug interactions with antimyeloma medications. Despite an overall improvement in survival in MM over the last two decades, outcomes for elderly patients have failed to keep pace with the improvements achieved in younger patients.52,53 A clear need exists for treatment of MM that is more efficacious and better tolerated in the older patients who make up the vast majority of the MM treatment pool, and novel agents look well equipped to provide this.
Important insights into the efficacy of novel agents in the elderly patient population can be gained from subgroup analyses of the clinical trial data. New IMiDs and proteasome inhibitors have all demonstrated improved PFS versus comparators in elderly patients, with similar efficacy results reported in younger patients.17 However, perhaps the most striking improvements in outcomes in the elderly were achieved with mAbs. In the phase III ELOQUENT-2 trial (Study of Lenalidomide and Dexamethasone With or Without Elotuzumab to Treat Relapsed or Refractory Multiple Myeloma), the benefit of adding elotuzumab to Rd was retained in patients aged 65 years or older, with mAb combination therapy actually achieving better hazard ratios (HRs) for PFS than in the younger patient cohort (HR 0.65 for patients aged ≥65 years versus 0.75 for patients aged <65 years).11 In the CASTOR study (Addition of Daratumumab to Combination of Bortezomib and Dexamethasone in Participants with Relapsed or Refractory Multiple Myeloma), similar findings were seen with daratumumab + Vd, where the positive impact on PFS was greater in elderly patients aged ≥65 years versus younger MM sufferers (HRs of 0.44 and 0.35, respectively).13 Emerging data also support the value of daratumumab monotherapy in older patients with RRMM, with almost a third of very elderly patients (aged ≥75 years) responding to treatment in the SIRIUS trial (Efficacy and Safety Study of Daratumumab in Patients with Multiple Myeloma Who Have Received at Least 3 Prior Lines of Therapy [Including a Proteasome Inhibitor (PI) and Immunomodulatory Drug (IMiD)] or Are Double Refractory to a PI and IMiD).40
From a practical perspective, the simplicity and convenience of ixazomib’s weekly oral dosing schedule, together with its favourable safety profile, highlight it as a good option for elderly or frail patients with an asymptomatic relapse. Mobility issues and access to transportation are key issues that must be considered when prescribing parenterally administered drugs delivered in a healthcare or hospital setting rather than at home.54 Carfilzomib may also be a preferred proteasome inhibitor choice over bortezomib in older patients with MM based on evidence from the phase III ENDEAVOR study (Carfilzomib and Dexamethasone Versus Bortezomib and Dexamethasone for Relapsed Multiple Myeloma Patients) in RRMM.41,42 Although carfilzomib was associated with a higher rate of cardiac AEs in very elderly patients in the trial, this was offset by its superior efficacy versus bortezomib and lower discontinuation rate among patients aged ≥75 years.55
Very elderly patients with myeloma, aged 80–85 years, are a group that is under-represented in clinical trials and which require careful management. In particular, it is important to adopt tailored treatment strategies for very elderly patients with myeloma and undertake dose modifications where required. Comprehensive geriatric assessment tools have been developed that can be employed to evaluate patients and guide management decisions in everyday practice. Major factors to consider when choosing treatment for very elderly patients include estimated survival, symptom burden, toxicity and impact on QoL of both the disease and its treatment.56
High-risk cytogenetics
High-risk cytogenetic features, such as translocations t(14;16) and t(14;20), chromosome 17p deletion [del(17p)] and gain in 1q21 have been shown to be independent markers of poor prognosis in MM.57 Despite a general improvement in outcomes across the MM arena, PFS and OS for patients with high-risk cytogenetics remains consistently poorer than their standard-risk counterparts.10,58,59 Clinical evidence indicates that novel agents can help to close the survival gap for patients with poor prognosis and cytogenetic abnormalities. When patients in the phase III TOURMALINE-MM1 study (Comparing Oral Ixazomib Plus Lenalidomide and Dexamethasone Versus Placebo Plus Lenalidomide and Dexamethasone in Adult Patients with Relapsed and/or Refractory Multiple Myeloma) were analysed by cytogenetic risk, ixazomib + Rd overcame the poor PFS associated with high-risk cytogenetic abnormalities, with limited additional toxicity. Median PFS was improved to 21.4 months with ixazomib + Rd therapy versus 9.7 months with Rd alone in high-risk patients (compared to 20.6 versus 15.6 months in the standard-risk group).57 This PFS benefit was consistent across individual cytogenetic abnormalities, including patients with del(17p). The tolerable all-oral ixazomib + Rd regimen may therefore provide a useful therapeutic approach for patients with high-risk cytogenetics which allows for prolonged active treatment, thereby enabling extended control of aggressive disease.57
Data from the pivotal phase III ASPIRE (Study Comparing Carfilzomib, Lenalidomide and Dexamethasone [KRd] versus Lenalidomide and Dexamethasone [Rd] in Subjects with Relapsed Multiple Myeloma) and ENDEAVOR trials indicate that carfilzomib is a more potent proteasome inhibitor than bortezomib for patients harbouring high-risk cytogenetic abnormalities; however, it is unable to completely negate the unfavourable impact on prognosis. PFS was significantly extended to 8.8 months with carfilzomib–dexamethasone versus 6.0 months for bortezomib–dexamethasone in patients with high-risk cytogenetic status in ENDEAVOR. However, outcomes remained superior in the standard-risk cytogenetic group compared to the high-risk group: not reached versus 10.2 months, respectively.60 In ASPIRE, a trend towards increased PFS with carfilzomib + Rd was noted versus Rd alone in patients with high-risk cytogenetic abnormalities.59 However, in the final analysis of ASPIRE, the PFS benefit of carfilzomib initially observed in patients with high-risk cytogenetic features was found not to convert into an OS advantage. The OS HR for these high-risk patients (1.08) differed considerably from that of standard-risk patients (0.74) in the overall ASPIRE population, and median OS was identical (36 months) for both carfilzomib + Rd versus Rd alone in the high-risk cohort.61
Elotuzumab and daratumumab have also demonstrated efficacy in patients with high-risk cytogenetic abnormalities and achieve comparable efficacy outcomes across all patient subgroups with MM. In ELOQUENT-2, elotuzumab produced HRs for disease progression ranging from 0.53–0.75 for patients with the high-risk cytogenetic features Del(17p) [cut-off of one cell only], 1q21 and t(4;14) versus an HR of 0.70 for the overall patient population.11 In the phase II ELOQUENT-3 trial, addition of elotuzumab to pomalidomide and dexamethasone reduced the risk of disease progression or death by 46% compared with pomalidomide and dexamethasone alone; median PFS was 10.3 months and 4.7 months, respectively.62 In the CASTOR and POLLUX (Study Comparing Daratumumab, Lenalidomide and Dexamethasone with Lenalidomide and Dexamethasone in Relapsed or Refractory Multiple Myeloma) studies, daratumumab improved PFS in high-risk patients in combination with both Rd or Vd.12,13 In a subgroup analysis of CASTOR based on cytogenetic status, PFS was significantly longer in patients with high-risk cytogenetics who received daratumumab + Vd versus Vd (HR: 0.46), with estimated 12-month PFS rates of 63.2% versus 26.7%, respectively.63 However, closer inspection of PFS data shows dips in the Kaplan-Meier curves after bortezomib treatment is stopped, potentially indicating that a substantial part of the activity seen with daratumumab + Vd in high-risk patients was driven by the proteasome inhibitor.63 Similarly, for patients in the 1–3 prior lines subgroup of POLLUX with high-risk cytogenetic features, significantly longer PFS was observed with daratumumb + Rd combination therapy compared to Rd (median: not reached versus 8.3 months; HR: 0.30) and significantly superior response rates were achieved in patients with high-risk cytogenetic status treated with DRd versus Rd, respectively.64
Triplet therapy with a proteasome inhibitor plus IMiD is already recommended by the International Myeloma Working Group (IMWG) for patients with high-risk cytogenetic status given the positive impact of such regimens on clinical outcomes in those with adverse cytogenetics. The IMWG further notes that this high-risk patient population should receive this active treatment as prolonged therapy until disease progression, given the real risk of rapid relapse in the absence of sustained disease suppression.23,57 In this regard, the all-oral ixazomib + Rd regimen may provide a useful therapeutic approach for patients with high-risk cytogenetics which allows for prolonged active treatment, thereby enabling extended control of more resistant disease.57 The myeloma mAbs also appear good treatment options for patients carrying high-risk cytogenetic abnormalities given the compelling efficacy evidence from clinical trials to date.
In addition to their patient-specific benefits, novel agents open up new therapeutic avenues and give greater flexibility and choice in the overall management of RRMM. A wider selection of IMiD and proteasome inhibitor agents gives clinicians more options when managing suboptimal response or poor tolerability and when switching between classes in single-refractory disease. The diversely different modes of action of the therapeutic mAbs can also help to circumvent mechanisms of drug resistance to previously used therapies and achieve clinical responses in double-refractory disease.17
Emerging treatments in relapsed, refractory multiple myeloma
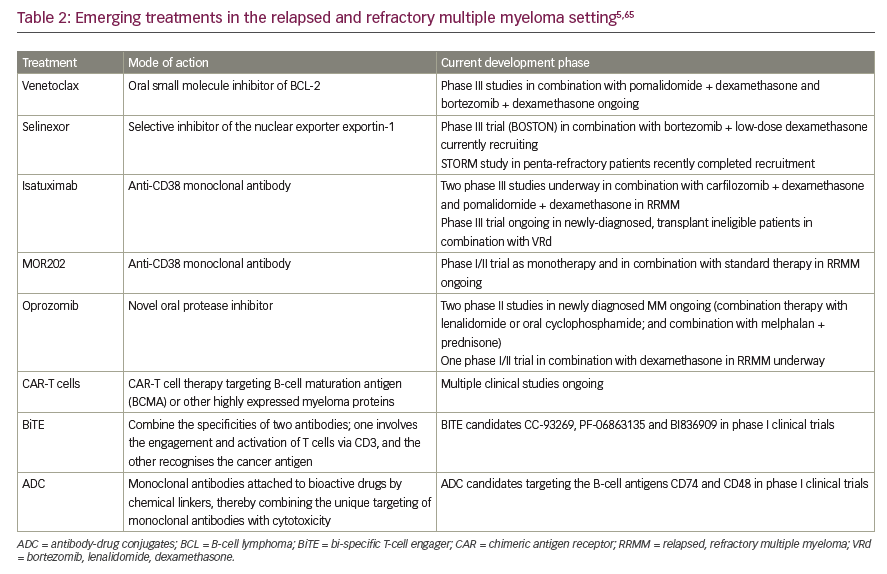
Looking to the future, the clinicians’ toolkit may be expanded further as several novel agents and new drug classes are undergoing active clinical development for RRMM.65 The histone deacetylase inhibitor, panobinostat, is already approved in Europe in combination with bortezomib and dexamethasone for the treatment of adult patients with RRMM who have received at least two prior regimens including bortezomib and an IMiD.66 Particularly promising drugs emerging from the clinical pipeline for myeloma include bi-specific T-cell engager (BiTE) antibodies, antibody–drug conjugates, chimeric antigen receptor-T (CAR-T) cells and the novel inhibitor selinexor (Table 2).5,65 It is beyond the scope of this review to examine investigational agents for MM in any detail but a few agents warrant special mention. BiTEs bind simultaneously to both malignant and immune cells by engaging with a target antigen expressed by the tumour cell, thereby triggering T-cell activation and an anti-cancer response. BiTEs targeting the B-cell proteins BCMA and CD3 have shown preclinical activity in MM.67 Selinexor is a first-in-class orally bioavailable selective inhibitor of nuclear export that specifically blocks XPO1, a transport protein which is overexpressed in myeloma. Through its mechanism of action, selinexor prevents the expression of oncoproteins by myeloma cells and therefore leads to selective apoptosis of malignant cells. Selinexor has shown encouraging activity in heavily pre-treated MM in early-stage clinical trials in combination with dexamethasone.68
Conclusion
Extensive research into novel agents has supported claims that they are ground-breaking additions to the existing MM treatment paradigm in RRMM, offering enhanced efficacy over doublet combinations with a manageable toxicity profile. As such, novel agent-based triplets have now been established as a standard of care in ESMO guidelines for relapsed or refractory disease. The near future also looks set to see the expansion of novel agents into both the first-line and maintenance settings in MM as clinical data from major ongoing clinical studies are released.
Novel agents have helped to overcome some of the key treatment challenges and address critical unmet needs in the RRMM arena including toxicity burden, adverse impact on QoL and the problems of drug resistance and clonal evolution. Perhaps most importantly, novel agents also offer the ability to tailor therapy to meet the needs of specific patient populations, including the elderly, unfit or frail patients, patients carrying high-risk cytogenetic abnormalities and those with toxicity-related complications and comorbidities. With the advent of novel agent-based regimens, clinicians now have the therapeutic tools to optimise current clinical practice in their management of RRMM and take the first key steps towards individualised therapy for patients.












