Acute lymphoblastic leukemia (ALL) is of B cell precursor lineage (BCP-ALL) or, less commonly, T cell precursor lineage (T-ALL), which comprise multiple subtypes defined by structural chromosomal alterations that are initiating lesions, with secondary somatic DNA copy number alterations and sequence mutations that contribute to leukemogenesis. Survival rates for ALL have steadily improved in children, such that more than 85% of children can expect to be cured with current cytotoxic therapies. Unfortunately, the same increases have not been demonstrated in adult patients. Despite high rates of complete remission (CR) (80–90%), the cure rates are only 40–50%, because of relapses.1 One approach to improving outcomes in adult ALL involves intensification of existing chemotherapy combinations or the addition of chemotherapeutic agents.2 The results of allogeneic hematopoietic stem cell transplantation (HSCT) for adults with ALL in first-line therapy have also improved significantly over time,3 but it is unlikely that the sole intensification of regimens can continue to improve prognosis substantially. Intensifying chemotherapy may reduce the incidence of resistance, but at the cost of increased toxicities. Outcomes remain particularly poor in relapsed/refractory (R/R) patients. Response rates after salvage treatment range from 18–45% and median survival times from 2–8 months, with less than 10% survival after 5 years.4–9 In this setting, allogeneic HSCT is the only curative option, but this can only be achieved in a subgroup of patients.4,5,8 A retrospective analysis of 1,706 adult patients with Philadelphia chromosome-negative R/R BCP-ALL from 11 study groups and large centers was recently conducted in order to provide detailed reference outcomes for this patient population.10 The overall CR rate after first salvage was 40%, ranging from 35–41% across disease status categories (primary refractory, relapsed with or without prior transplant), and was lower after second (21%) or further (11%) salvage. The 3-year survival rates range from 2–5%.
Thus, new treatment options are needed for this patient population. This, combined with a better understanding of disease pathogenesis has led to major advances in drug development and reassessment of risk stratification.11 The landscape of treatment is currently rapidly changing, ranging from the application of precision genomic medicine in subsets of ALL to the delivery of novel immunotherapeutic approaches. Emerging therapies in the frontline and salvage settings offer the promise of improved response rates and opportunities for long-term survival, with the potential for less toxicity than chemotherapy.12–14
In this review, we will discuss the use of monoclonal antibodies and novel immunotherapeutic approaches in ALL, including bispecific T cell-engaging molecules and chimeric antigen receptor (CAR) T cells.
Antibodies and immunoconjugates
B-lineage blast cells express a variety of specific antigens, such as CD19, CD20, and CD22. Monoclonal antibodies have been developed to target these antigens.15,16 The B-lineage surface antigen CD19 is expressed during B cell development and expressed on the surface of more than 90% of BCP-ALL blasts. CD20 is expressed on B-precursor leukemic cells in up to 50% of patients. CD22 is expressed on B-precursor leukemic cells in more than 90% of patients. Monoclonal antibodies targeting these cell surface markers represent one of the most exciting groups of compounds under investigation in the treatment of adults with ALL (Table 1).
There are major variables influencing the success of monoclonal antibodies: the rate of internalization; the high expression of the target antigen on the malignant cells, the high density of antigen expression on the malignant cells, the lack of expression on normal tissues; the linker chemistry, and the intracellular as well as intracellular stability; and the selection of payload for the malignant cell.
Anti-CD19 monoclonal antibodies
Nearly all cases of BCP-ALL display a bright expression of CD19, making this marker an ideal candidate for target therapy. Initial poor results have hampered the development of naked anti-CD19 compounds.17 Denintuzumab mafodotin (SGN-CD19A) is a humanized anti-auristatin F, a microtubule-disrupting agent, which has recently been investigated in a phase I study in R/R BCP-ALL patients. A preliminary analysis showed a 30% response and achievement of complete MRD responses.18 Combotox is a mixture of antibodies targeting both the CD19 and CD22 antigens, conjugated with a deglycosylated ricin A chain, which shows transient responses in a phase I study19 and is currently evaluated in combination with chemotherapy for the treatment of R/R BCP-ALL. Coltuximab ravtansine (SAR3419) is a humanized anti-CD19 antibody conjugated with maytansin, a potent microtubule inhibitor. Although associated with about 25% of responses, the study was prematurely discontinued.20 New hints in terms of anti-CD19 are coming from the development of blinatumomab, a first-in-class bispecific T cell engager (BiTE®) antibody, that engages cytotoxic CD3+ T cells and drives them in contact with CD19+ cells (see T cell-engaging therapies).21
Anti-CD20 monoclonal antibodies
The anti-CD20 monoclonal antibody rituximab has substantially improved the outcome of patients with Burkitt leukemia/lymphoma. With repeated short cycles of intensive chemotherapy combined with rituximab, the overall survival of this subset of patients increased from 60% to up to 80%.22,23 In BCP-ALL, combining cyclophosphamide, vincristine, Adriamycin® (Pfizer), and dexamethasone (Hyper-CVAD) plus rituximab gave results significantly better than those observed with chemotherapy alone.24 These results were confirmed by the German group25 and, more recently, by the randomized study from the French group, which showed a significant advantage with chemotherapy combined with rituximab with a 2-year overall survival (OS) of 71% versus 52% and a 2-year event-free survival (EFS) of 65% versus 52%.26 In this phase III trial, rituximab was combined with the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) 2005 frontline chemotherapy regimen. Rituximab was given in all treatment phases (16–18 infusions). Adding rituximab to standard chemotherapy should therefore be the standard of care for these patients, although the optimal dose schedule remains to be determined. Studies are underway to continue to optimize the incorporation of anti-CD20 into ALL treatment regimens. Combination of chemotherapy with other anti-CD20 has also been tested. Ofatumumab combined with Hyper-CVAD showed 97% of CR and 67% of minimal residual disease (MRD) negativity after induction and a 2-year OS of 87%.27

Anti-CD22 monoclonal antibodies
Inotuzumab ozogamicin (CMC-544) links a humanized anti-CD22 antibody to the chemotherapeutic agent calicheamicin, a cytotoxic antibiotic agent. CD22 is expressed by more than 69% of leukemic blasts in patients with B cell lineage ALL. It is expressed on B cells during maturation, lost on differentiation to plasma cells, and not expressed on hematopoietic stem cells or other normal tissues. After the conjugate binds to CD22, the CD22-conjugate complex is rapidly internalized, and calicheamicin is released.28 Calicheamicin binds to the minor groove of DNA and thus induces double-strand cleavage and subsequent apoptosis.29 In a phase II study, inotuzumab showed a CR rate of 66% in R/R ALL patients. Among remitters, 78% achieved a complete molecular response.12,30 In the pivotal INO-VATE (NCT01564784) randomized phase III study comparing inotuzumab to standard chemotherapy, complete response was obtained in 81% versus 33%, respectively.31 MRD-negativity in remitters was observed in 78% of cases versus 28%. Inotuzumab was given at 0.8 mg/m2 on day 1, 0.5 mg/m2
on days 5 and 15 of a 21–28 day cycle. Hepatic toxicity was similar in all age groups. Venous occlusive disease (VOD) was more common in older than in younger patients undergoing HSCT following inotuzumab administration (33% versus 17%, respectively). Five of the 15 cases of VOD developed shortly after treatment, 10 developed after transplantation (median: 16 days after transplantation). Inotuzumab was also combined with low-dose chemotherapy in untreated BCP-ALL elderly patients.32 Inotuzumab was administered at single dose of 1.3 to 1.8 mg/m2 with each of the first four courses. In the mini-Hyper-cyclophosphamide, vincristine, dexamethasone (CVD) inotuzumab ± rituximab, the overall response rate was 97%, MRD-negativity was 100%, and the 2-year OS was 64%. The 3-year OS was 52% and compared favorably to an historical cohort treated by Hyper-CVAD (3-year OS: 36%). Grade 3–4 non-infectious toxicities occurred in 20% of patients. VOD was observed in 11% of patients. The United States Food and Drug Administration (FDA) has recently approved inotuzumab ozogamicin as monotherapy for the treatment of adults with R/R CD22-positive BCP-ALL.

Epratuzumab is a humanized monoclonal antibody targeting the CD22 B cell differentiation antigen.33 After binding to CD22, the receptor/antigen complex is internalized. Its predominant anti-tumor activity is mediated through antibody-dependent cellular cytotoxicity. In adult ALL, a phase II study showed an improved response rate in relapsed/refractory ALL patients when giving epratuzumab combined with cytarabine and clofarabine.34 Epratuzumab was given intravenously weekly for four doses, starting on day 7 of therapy. The response rate was 52%, significantly higher than a historic control without epratuzumab (17%). However, only one patient on day 6, who had MRD assessed, became MRD negative. A phase II trial of epratuzumab combined with vincristine and dexamethasone in older patients with relapsed/refractory B cell lineage ALL showed a 40% response, and a median OS of 4 months.35 The most common adverse events were mild to moderate infusion reactions. Combining epratuzumab with Hyper-CVAD in very high-risk R/R ALL patients provided an overall response rate of 50%.36 Almost half of the evaluated responders achieved a negative MRD status. Anti-CD22 fractionated radioimmunotherapy with Yttrium-90 (90Y) epratuzumab tetraxetan has been recently evaluated.37 Results showed that 90Y-labelled epratuzumab was well tolerated. The recommended dose was 2 x 10.0 mCi/m2 one week apart per cycle. Similarly, epratuzumab linked to a topoisomerase I inhibitor (epratuzumab-SN-38) was recently tested, demonstrating activity against B cell leukemia cell lines.38
Anti-CD38 monoclonal antibodies
Isatuximab, also referred to as SAR650984, is a naked immunoglobulin G1 monoclonal antibody that selectively binds to the human cell surface antigen CD38.39 CD38 is a 45 kDa membrane protein expressed in a number of hematological malignancies from B-lymphocyte, T-lymphocyte, and myeloid origin (Table 1). The biological mechanisms by which isatuximab can affect the killing of CD38-postive cells involve the antibody-dependent cellular-mediated cytotoxicity (ADCC), the complement-dependent cytotoxicity (CDC), and the induction of apoptosis. The development of T cell-directed monoclonal antibody therapies are lagging and antibody treatments are missing compared with BCP-ALL. For this reason, isatuximab is currently evaluated for its safety profile and efficacy in a phase II trial for ALL of T cell origin with an administration every week for 4 or 8 weeks (ISLAY) (NCT02999633).
Anti-CD52 monoclonal antibodies
Alemtuzumab is a humanized monoclonal antibody against CD52. CD52 is a membrane glycoprotein expressed by 70–80% of both BCP-ALL and T-ALL (Table 1). Alemtuzumab has only been evaluated in small trials, with results that have been somewhat disappointing. No CR was observed in six patients with R/R ALL receiving 30 mg three times weekly for 4–12 weeks.40 In untreated patients, alemtuzumab was tested in 11 patients in morphological CR in order to lower post-remission MRD levels. Only a one-log median MRD reduction was obtained and a drastic reduction of lymphocytes predisposed patients to opportunistic infections.41
T cell-engaging therapies
The immune system could play a key role in the treatment of malignancies. This is the basis for the graft-versus-leukemia effect, which contributes in part to the efficacy of HSCT42 and is the rationale for donor lymphocyte infusion.43 This has led to the increasing study of appropriate targets for immunotherapy, including tumor-specific and/or tumor-associated antigens. These can be attacked by different cellular components of the immune system, including T cells, natural killer (NK) cells, and dendritic cells.
Bispecific T cell engager and dual-affinity re-targeting antibody constructs
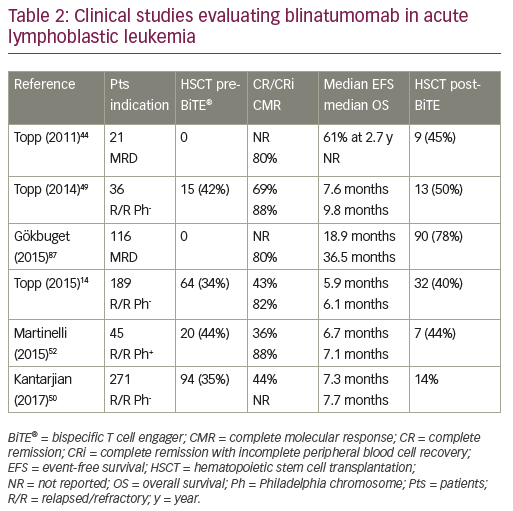
While unconjugated anti-CD19 monoclonal antibodies have not induced measurable anti-tumor effects, bispecific anti-CD19 monoclonal antibodies have shown impressive single-agent activity in ALL.44 Blinatumomab, a BiTE antibody, directly recruits effector T cells to augment the anti-leukemic effect (Figure 1). Blinatumomab contains the variable domains of a CD19 antibody and a CD3 antibody, which are joined by a non-immunogenic linker. Blinatumomab binds simultaneously to CD3-positive cytotoxic T cells and CD19-positive B cells. CD19 is expressed by about 100% of leukemic cells in patients with B cell lineage ALL. It is expressed on B cells during an early stage of maturation, lost on differentiation to plasma cells, and not expressed on hematopoietic stem cells or other normal tissues.45 On binding to CD19, endogenous cytotoxic T cells become activated and give activation signals that promote their proliferation,46 and then induce leukemia blast death via the pore-forming perforin system.47 Blinatumomab has a half-life of 2–3 hours: administered by 28-day continuous infusion followed by a 14-day rest period. The main results are summarized in Table 2. It has been initially explored in patients in morphological remission with MRD+ ALL and successfully converted the majority of patients to a MRD− status.44,48 Single-arm studies demonstrated the efficacy and safety of blinatumomab in the treatment of R/R BCP-ALL.14,49 In MRD-positive ALL, blinatumomab allowed a switch to MRD-negativity in 80% of cases.44 In adult patients with R/R ALL, the response rate was 43% and the MRD response rate in remitters was 82%.14 The rate of complete MRD response did not differ significantly across baseline age, sex, line of treatment, and leukemic burden categories. This led to the approval of blinatumomab by the FDA for the treatment of R/R ALL. Most frequent reported adverse events were grade 3/4 reversible neurological events (17–19%).44,49 In a multicenter, randomized phase III study (TOWER study [NCT02013167]) that compared the outcomes of patients with R/R ALL treated with blinatumomab versus standard of care chemotherapy, the median OS was 7.7 months and 4.0 months, respectively, and 6.9 months and 3.9 months when censoring at the time of HSCT. The rate of CR was 34% versus 16%. In this study, blinatumomab was given at the dose of 28 μg/day (9 μg/day on days 1–7 of cycle 1). EFS and remission duration were also longer with blinatumomab, while there were lower incidences of myelosuppression, and neurologic events and cytokine release syndrome potentially observed with blinatumomab were generally mild to moderate.50 Long-term survival after blinatumomab treatment has been shown to be associated with an MRD response and potentially with a higher degree of T cell expansion.51
Alternative therapies could be proposed to patients who remain positive for BCR-ABL transcripts more than 2 months after starting imatinib therapy after transplant. Blinatumomab has showed promising results in patients with high-risk ALL.44 Activity has been demonstrated in Philadelphia chromosome-positive (Ph)-ALL with T315I mutation.52 CR was achieved in 36% of cases, and in 40% of patients with mutation T315I. Preliminary results indicate that treatment with blinatumomab is able to convert MRD-positive ALL into MRD-negative status. Eighty-eight percent of patients achieved a complete MRD response.52
The first-in-class anti-CD19/CD3 BiTE, blinatumomab, has been approved for the treatment of R/R Ph-negative BCP-ALL. Adverse events are acceptable and consistent with known pharmacological effects of T cell engagement. However, optimal position of blinatumomab in the ALL treatment paradigm is currently unknown and studies in the front-line setting are ongoing.
Other T cell-engaging antibody constructs
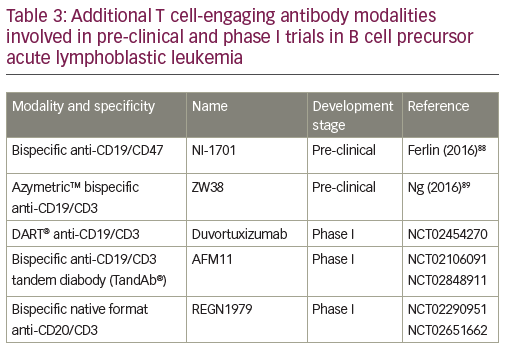
The success of the BiTE format triggered the search for other bispecific antibody formats of similar size and valence.53 Besides the monomer bivalent BiTE format, other structural bispecific antibody constructs are in development: heterodimer bivalent dual affinity re-targeting (DART®),53,54 homodimer tetravalent derivative (TandAb®),55,56 heterotetramer bivalent native format bispecific antibody.57,58 A range of these additional T cell-engaging antibody modalities are currently in the pre-clinical phase or in phase I trials (Table 3). The DART format is based on the diabody format that separates cognate variable domains of heavy and light chains of the two antigen-binding specificities on two separate polypeptide chains.59 An in vitro side-by-side comparison of CD19xCD3 DART and BiTE molecules showed that the bispecific antibody in the DART format consistently outperformed the BiTE format with respect to the maximal level of B cell lysis, the concentration required for half-maximal B cell lysis, and the induction of molecular markers for T cell activation.54 DART format revealed a higher association rate for CD3, a lower dissociation rate for CD19, and an ability to cross-link T cells and B cells more efficiently.
A first-in-human phase I study of humanized anti-CD19/anti-CD3 DART (duvortuxizumab) is currently ongoing (NCT02454270). AFM11 is a dimeric tetravalent derivative (TandAb) molecule, which recruits immune cells to tumors. It binds to CD3 on T cells and mediates simultaneous binding to CD19 on malignant cells.60 It is currently being investigated in a phase I trial in R/R BCP-ALL (NCT02848911).
Chimeric antigen-receptor T cells
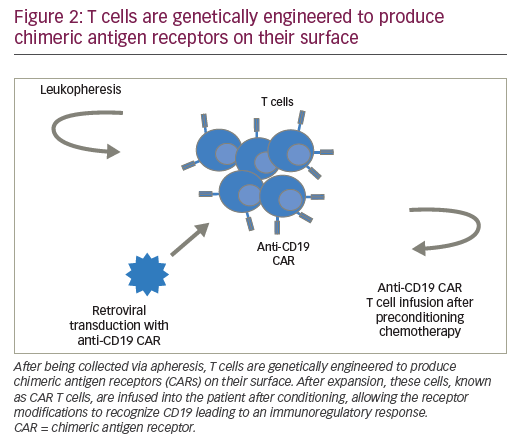
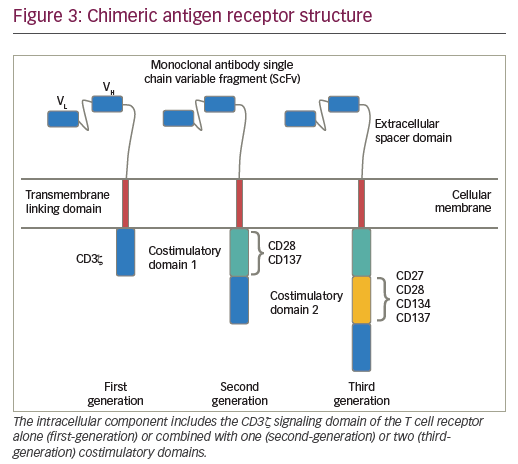
CARs are hybrid receptor constructs that contain a target recognition domain linked to an intracellular component that activates a signaling cascade in the immune effector cell. Normal autologous or allogeneic T cells can be harvested from patients or normal donors to be genetically modified to express CAR, recognizing specific targets on leukemic cells, then expanded and reinfused to exert antileukemic activity (Figure 2). CARs are synthetic receptors involving several key components: an extracellular major histocompatibility complex (MHC) independent antigen binding domain derived usually from a monoclonal antibody single chain variable fragment (ScFv); an extracellular spacer domain; a transmembrane linking domain; and an intracellular costimulatory T cell signaling domain or multiple domains. DNA constructs encoding such CARs may be stably incorporated into T cells via retroviral or lentiviral transduction. Retro- or lentiviral approaches have the advantage of long-term gene expression with long-term disease control from a single infusion of engineered T cells. A non-viral system (so-called “sleeping beauty”) consists in transducing T cells with the gene of interest through the use of transposase and DNA plasmid.61 T cells engineered to express such CARs engage an antigen on a tumor cell through the extracellular antibody domain, activating the T cells in a MHC-independent manner. Various generations of CARs have been designed (Figure 3). Most first-generation CARs utilized CD3ζ intracellular signaling domain to activate T cells, while subsequent generations of CARs have optimized ScFv and linker component design and have incorporated additional intracellular costimulatory domains (CD28 or 4-1BB in second-generation CARS; a combination of CD27, CD28, 4-1BB, ICOS, or OX40 in third-generation CARs) in efforts to increase the expansion, persistence and potency of CAR T cells, and to prevent cellular exhaustion in vivo.62,63
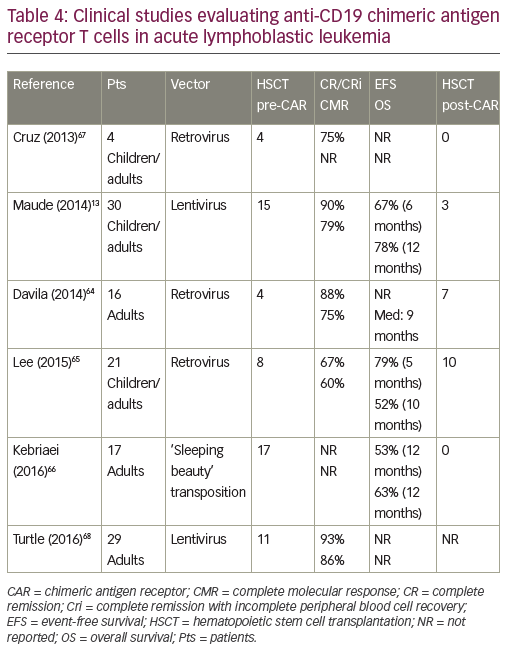
CD19-directed CAR-modified T cells are the most advanced engineered T cells presently used for ALL. Studies using CD19 CAR designs have been performed by several groups and showed CR rates ranging from
70–90% (Table 4).13,64–68 These studies included patients with a prior history of allogeneic HSCT. No graft-versus-host disease was observed, but persistence of CAR-modified T cells was very heterogeneous according to CAR designs. Similar responses were observed in high and low disease burdens.69 Persistence of 19-28z CAR T cells was reported to be 1 to 3 months.64 That of CD19-CAR T cells (CD28 costimulatory domain) was up to 68 days with rapid B cell recovery,65 while CTL019 T cells (4-1BB costimulatory domain) persistence was 68% at 6 months with a B cell aplasia longing at up to 2 years.13 T cells engineered to express a CD19-directed CAR therefore have the potential to produce durable remissions. However, more mature follow-up is needed across studies.
CD22 is highly expressed on the majority of ALL cells. Therefore, there is a substantial interest in generating a CAR capable of targeting CD22 in BCP-ALL. CAR T cells targeting CD22 or CD19/CD22 are under investigation.70
Target identification for T cell lineage ALL poses a particular challenge as blastic cells express the same antigens as normal T cells. CAR therapy may therefore not be possible in this leukemia subset.
Cytokine release syndrome
Cytokine release syndrome (CRS), resulting from the high magnitude of immune activation by T cell-engaging immunotherapies, is a reversible on-target toxicity manifested by high fever with myalgias and potential progression to life-threatening capillary leak with hypoxia and hypotension.13,65 Both blinatumomab and anti-CD19 CAR T cell therapy have treatment-related events.71 The etiology of central nervous system toxicity remains unclear. Disease burden in ALL strongly correlates with the severity of CRS. The infusion dose of anti-CD19 CAR T cells may have some effect on the severity of CRS.71 CRS is correlated with T cell proliferation and efficacy, and high levels of cytokines, including interleukin-6 (IL-6), but also tumor necrosis factor (TNF)α, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interferon (IFN)γ. With blinatumomab administration, CRS has been prevented with corticosteroid premedication, disease cytoreduction, and dose adjustments.50 After CAR T cell administration, CRS has been reported from 27% to 43% of cases (Table 5).13,64,65,69 CRS occurs within 1 to 14 days of anti-CD19 CAR T cell infusion. The interleukin-6 receptor (IL-6R) blocking antibody tocilizumab was used as part of the management of severe CRS (generally for grade 4 CRS).69
Allogeneic natural killer cell therapy
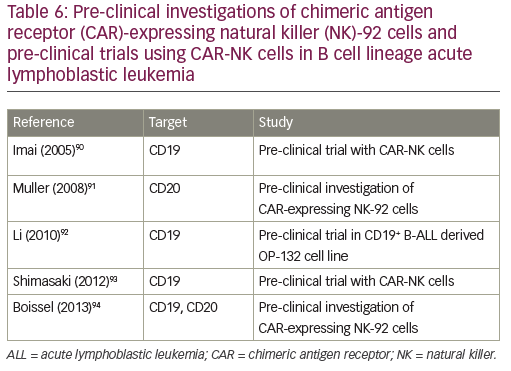
Natural killer (NK) cells play a major role in the surveillance and elimination of malignant cells, and might be used in the treatment of leukemia. Their activity depends upon activating and inhibitory receptors, as well as modulating coreceptors. Adoptive transfer of mature allogeneic NK cells in the nontransplant or transplant setting has also been shown to be safe and feasible, and its efficacy in humans is under investigation.72 Two approaches are currently in development: the adoptive transfer of NK cells from an alloreactive donor with a moderate lympho-depleting chemotherapy to induce homeostatic lymphocyte proliferation with transient expansion of the transferred allogeneic NK cells without the establishment of a permanent donor hematopoiesis;73 and the transplant of hematopoietic stem cells after myeloablative therapy with the permanent engraftment of donor NK cells, establishing the donor killer immunoglobulin-like receptors (KIR) phenotype in the patient.74 Allogeneic NK cells exert anti-leukemic effects without causing graft-versus-host disease. NK activity can be augmented using cytokines. NK cell therapy can be further improved by overcoming the killer immunoglobulin-like receptor (KIR) KIR ligand (KIRL) mediated inhibition of NK cells: KIR–KIRL interaction can be blocked by a monoclonal antibody, which abolishes the inhibitory signal;75 KIR–KIRL-mediated inhibition can be overridden by the activation of the Fc-receptor (CD16), with an antibody directed against an antigen (CD19) expressed on leukemic cells;76 a bispecific (CD16xCD19) or a trispecific (CD16xCD19x CD22) killer engager can activate NK cells via the Fc-receptor against antigens expressed on leukemic blasts;77,78 CAR-NK cells can also be directed against the CD19 antigen.79 Pre-clinical investigations of CAR-expressing NK-92 cells and pre-clinical trials using CAR-NK cells in B cell lineage ALL are indicated in Table 6. Clinical trials using anti-CD19 haploidentical CAR-NK cells (NCT00995137, NCT01974479) or allogeneic redirected NK 92 cells (NCT02892695) are currently ongoing.
Conclusions
Treatment of relapsed ALL remains a considerable challenge. Substantial numbers of adult patients do not successfully reach a second CR with standard chemotherapy and therefore are not candidates for allogeneic HSCT, which represents, at that stage, their only chance of a potential cure. Novel immunotherapy represents a major improvement in the treatment of R/R BCP-ALL, with achievement of high rates of CR and molecular complete response. Among monoclonal antibodies, two agents are particularly promising: blinatumomab, an anti-CD19/CD3 BiTE antibody, and inotuzumab ozogamicin, an anti-CD22 chalicheamicin-conjugated antibody. Both are effective in determining complete MRD clearance in a substantial number of patients. However, the median duration of response and OS remains far from optimal, with potential fast relapses even in patients with deep levels of molecular response. This type of immunotherapy was therefore currently considered as a bridge to transplant more than a valid alternative to HSCT. Incorporation of these agents in front-line therapy, concomitantly or sequentially, should improve the rates of MRD negativity and perhaps reduce the need for intensive therapies. Blinatumomab and inotuzumab ozogamicin have recently been FDA-approved for the treatment of R/R BCP-ALL. Although targeting a different antigen, these novel therapeutic approaches are targeting approximately the same subset of ALL patients. However, differences in their mechanisms of action suggest a possible immediate combination with chemotherapy for inotuzumab, while blinatumomab administration should not be used concomitantly with chemotherapy in order to preserve the effectors. Leukemic burden at the time of treatment could also be an important issue in the choice of the best monoclonal antibody to use. Immunotherapy theoretically implies a better efficacy in case of low malignant cell burden. In this setting, blinatumomab should find a better indication than inotuzumab for molecular relapses or reemergence of mixed chimerism after allogeneic HSCT, while inotuzumab should be a better choice in cases of high leukemic burden and proliferative disease. Another type of immunotherapy involves the adoptive transfer of T cells that have been genetically modified with a CAR to target leukemic cells. The early-phase clinical trials with CD19-targeting CAR T cells revealed efficacy with durable remissions in R/R BCP-ALL to standard salvage chemotherapy. However, it remains to be seen whether CAR therapy can translate into long-term cure and whether additional consolidative therapy, such as HSCT is necessary. Optimizing CAR therapy to a point where this treatment could abrogate the need for allogeneic HSCT would represent a real advance in the treatment of adult ALL.












