Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most-common neoplasm globally with ~600,000 cases diagnosed annually. The past 20 years have yielded a wealth of clinical trial data and this has transformed the standards of care for treatment of SCCHN. Historically, poor local disease control and overall survival have provided impetus for a multitude of clinical trials evaluating multimodality therapy in patients with SCCHN. The intricate anatomy and the critical functional and social roles of the head and neck region have no doubt also motivated significant efforts to identify alternatives to oncologic resection of malignant tumors in this region. In this review, we summarize the historical development of combined chemoradiation therapy in the treatment of SCCHN, provide an update of recent literature in this field, and discuss contemporary clinical questions that future studies may resolve.
Concurrent Chemoradiation—From Experimental to Preferred Treatment
Clinical investigation into the role of combined chemoradiation treatment for SCCHN dates to at least the late 1960s, with early studies exploring sequential and concurrent use of single agents including fluorouracil (5FU), bleomycin, cisplatin, methotrexate, and mitomycin.1 Such trials demonstrated poor response to single agent chemotherapy alone while pointing to cisplatin as the most effective single-agent with a 25–30% response rate.2–4 Subsequent studies explored the role of multi-agent chemotherapy and demonstrated marked improvement in disease response to 70% with combined cisplatin and bleomycin5 and >90% response rates with cisplatin and continuous infusion 5FU.6,7 Importantly, these early studies pointed to a potential survival benefit from the addition of chemotherapy to traditional definitive treatments of radiation and/or surgery for SCCHN and prompted further investigation in larger randomized phase III trials.

With refinement of chemotherapeutic regimens, consideration turned to the potential for definitive and organ-sparing chemoradiation approaches in patients with locally advanced SCCHN. This work was initially advanced by a series of single-institution studies demonstrating viability of definitive chemoradiation as an alternative to surgery for glottic and supraglottic laryngeal tumors.8–10 The historically important Veteran Affairs Laryngeal Cancer Study ultimately demonstrated that induction chemotherapy (ICT) followed by radiation could achieve organ preservation in SCCHN patients without compromise in overall survival.11 This trial established a rationale for the landmark Radiation Therapy Oncology Group (RTOG) 9111 study, which addressed the appropriate sequencing of chemotherapy and radiation in patients with locally advanced supraglottic or glottic larynx cancer. Patients were randomized to: ICT followed by radiation; concurrent chemoradiation (CCRT); or radiation alone.12,13 The results of RTOG 9111 demonstrated superiority of CCRT in terms of locoregional disease control and larynx preservation while no difference was observed in overall survival among the three arms. Subsequently, the Groupe d’Oncologie Radiothérapie Tête Et Cou (GORTEC) 94-01 trial randomized patients with locally advanced oropharyngeal primary tumors to radiation or CCRT with carboplatin/5FU and demonstrated improved local disease control as well as overall survival.14,15 Similar improvements in survival and locoregional control have been reported for nasopharyngeal patients treated with CCRT.16-21 A report from the Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACHNC) has suggested an overall survival benefit of 6.5% at 5 years with CCRT, but not with ICT or adjuvant chemotherapy.22,23 The effect of chemotherapy on survival in this meta-analysis was observed to decline with age for those older than 50 years with no demonstrable benefit shown in those 71 years or older. Collectively, although these studies differ in inclusion and exclusion criteria, clinical endpoints, and treatment methodologies (see Table 1), they confirm a benefit of CCRT versus radiotherapy alone as a definitive treatment for locally advanced SCCHN.
Induction Chemotherapy Preceding (Chemo) Radiation—A Viable Strategy?
Although a head-to-head comparison with CCRT indicated inferiority of ICT in terms of local disease control in the RTOG 9111 clinical trial,12 there remains interest in this therapeutic approach. Data from RTOG 9111 indicate equivalence of these approaches in terms of high-grade toxicities, making it difficult to justify the choice of ICT on grounds of patient tolerance. Nevertheless, follow-up studies to RTOG 9111 have sought to develop upon the platform of ICT in order to make this a more viable treatment option. The EORTC 24971/TA X 323 trial24,25 demonstrated improved overall survival with the addition of docetaxel (TPF) to the cisplatin and 5FU (PF) ICT regimen used in the RTOG 9111 study in patients with unresectable SCCHN. The TA X 324 trial similarly evaluated TPF versus PF in the setting of CCRT and substituting weekly carboplatin for cisplatin in these regimens. This study demonstrated improved locoregional and distant disease control as well as improved overall survival with TPF compared with PF.26 An additional follow-up study (GORTEC 2000-01) in locally advanced laryngeal and hypopharyngeal cancer confirmed improved disease response and larynx preservation with induction TPF compared with PF, but did not demonstrate any effect on survival.27 Although these studies differ in terms of inclusion and exclusion criteria, clinical endpoints, and treatment methodologies (see Table 1) they collectively support the superiority of TPF over PF-based ICT. Nevertheless, direct comparison of ICT with standard-of-care cisplatin-based CCRT is lacking.
The MACH-NC meta-analysis, as previously noted, did suggest inferiority of ICT compared with CCRT in terms of overall survival and locoregional disease control.22 It is noteworthy, however, that the benefit of ICT was more pronounced than that of CCRT in terms of rates of distant metastasis.22 This may reflect a distinct role of chemotherapy in these treatment approaches. Concurrent chemotherapy may play a predominant radiosensitizing role, resulting in a dominant effect on local disease control while multi-agent ICT may provide an effective systemic treatment for micro-metastatic disease. This hypothesis-generating observation suggests a potentially complementary nature of these treatments and has prompted interest in their combination. Thus far, efforts to examine the combination of ICT and CCRT have resulted in poor accrual and inconclusive findings (DeCIDE and PARADIGM studies).28,29 An ongoing randomized phase III study is comparing ICT with PF or TPF followed by cisplatin-based CCRT versus CCRT alone as first-line treatment for locally advanced SCCHN (NCT00261703). Results from the above studies on CCRT and ICT are also summarized in Table 1.
Adjuvant Chemoradiation
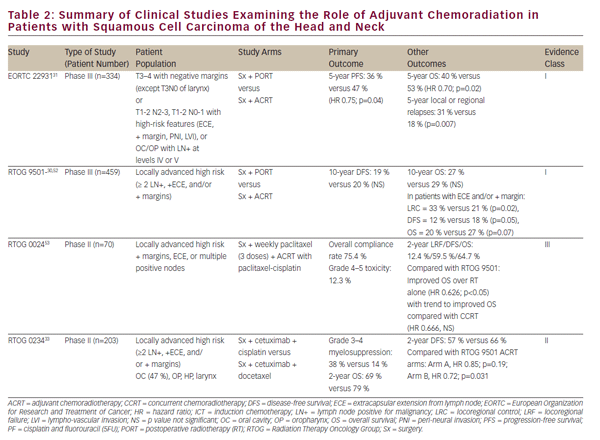
The aforementioned clinical trials have established CCRT as a viable definitive treatment for organ preservation in locally advanced SCCHN. Nevertheless, definitive oncologic resection remains an important treatment option for localized disease and a vital part of multimodality therapy for advanced tumors of the oral cavity, larynx, and hypopharynx. The role of chemoradiation in the adjuvant setting following oncologic resection has been largely defined by two phase III randomized trials— EORTC 22931 and RTOG 950130,31 (see Table 2). These studies examined the use of postoperative radiation versus adjuvant chemoradiation with cisplatin given every 3 weeks for three cycles in postoperative SCCHN patients. Both studies demonstrated improvement in locoregional disease control and progression-free survival while the EORTC study also showed an improvement in overall survival. A post-hoc analysis of these two studies suggested that the benefit of adjuvant CCRT was greatest in those with positive surgical margins and/ or extracapsular extension (ECE) from resected lymph nodes.32 The role of adjuvant CCRT in patients with close surgical margins is less clear and was supported by EORTC 22931, which included patients with ≤5 mm surgical margins. On the other hand, RTOG 9501 included patients based on positive but not close margins. Post-hoc analysis has suggested no apparent benefit of CCRT for patients meeting entry criteria for only one of these studies.32 In a similar vein, adjuvant CCRT has not been adequately studied in elderly patients as those over 70 years of age were excluded from EORTC 22931 and comprise only a small minority (5%) of patients in RTOG 9501.30,31
Pursuant to EORTC 22931 and RTOG 9501, adjuvant CCRT has now become standard-of-care treatment in high-risk postoperative SCCHN patients. The phase II RTOG 0234 study has explored alternative adjuvant chemoradiation treatment regimens, randomizing patients with multiple involved lymph nodes, ECE, or positive margins to the epidermal growth factor receptor (EGFR) antibody, cetuximab, plus docetaxel or cetuximab plus cisplatin. This study demonstrated an apparent survival benefit from concurrent adjuvant cetuximab and docetaxel.33 The results of this intriguing phase II study require validation in a phase III study prior to incorporation in clinical practice.
Radiotherapy Fractionation Impact on Chemoradiation
In an effort to heighten efficacy, prior clinical studies have investigated alternative fractionation schemes for radiotherapy administration with or without concurrent chemotherapy in SCCHN patients. The RTOG 9003 and the Danish Head and Neck Cancer Group (DAHANCA) trials demonstrated improvement in locoregional disease control with accelerated or hyperfractionated radiotherapy regimens when radiation is used alone.34,35 A subsequent meta-analysis has even suggested a modest survival benefit from hyper-fractionation of radiation when used as a sole treatment modality.36 In the setting of concurrent chemotherapy, however, the RTOG 0129 and the GORTEC 99-02 studies have demonstrated no improvement in clinical outcome with utilization of accelerated versus conventional radiotherapy.37,38 Hyper-fractionated CCRT, compared with hyper-fractionated radiation alone, resulted in improved locoregional disease control in a single institution study from Duke University and a phase III study from Yugoslavia, with the latter also showing improved overall survival and distant metastasisfree survival.39,40 These studies indicate that the benefit of CCRT persists with hyper-fractionated radiotherapy. A direct comparison of conventionally fractionated CCRT and hyper-fractionated CCRT has not been undertaken to date. It is notable, however, that the MACH-NC meta-analysis did not detect any difference in outcome among patients treated with CCRT using conventional or altered fractionation techniques.22
Toxicity—The Cost of Chemoradiation
In agreement with many single-institution studies that preceded it, RTOG 9111 indicated that the benefits of chemotherapy, whether administered sequential or concurrent with radiation, come at the expense of additional toxicity.12 Similarly, in the adjuvant setting, both the EORTC 2291 and RTOG 9501 clinical trials demonstrated an approximate doubling of grade 3 toxicities with chemoradiation compared to radiation alone.30,31 Such a trend persists even in the setting of hyper-fractionated radiotherapy.39 Although the expected rate of any specific toxicity as determined from historical studies is likely overestimated in the current era of highly conformal radiotherapy techniques, such as intensity modulated radiotherapy (IMRT). Two small, randomized trials have reported significant reduction in the incidence and severity of xerostomia with use of IMRT compared with conventional three-dimensional conformal radiotherapy for head and neck cancers (Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer [PARSPORT] and Tata Memorial Hospital [TMH] trials).41,42 Most recent trials investigating CCRT allow IMRT-based treatment delivery as a standard approach in management of SCCHN. It remains to be seen what impact IMRT will have on toxicity rates when combined with chemotherapy in these larger randomized studies.
Improved radiotherapy techniques coupled with increasing importance and availability of supportive care and ancillary services such as speechswallow pathology and dental care does limit the incidence of serious non-hematologic toxicities even further. However, the toxicity anticipated from CCRT is still expected to be higher than radiotherapy alone. This persistent toxicity reflects the clinical ‘cost’ of combining chemotherapy and radiation. In this setting, it remains paramount that physicians comprehensively evaluate patients to determine that the potential benefits of CCRT justify this cost. Use of single modality radiotherapybased treatments in patients with marked co-morbidities/low baseline performance status or with low-risk, early-stage disease may be reasonable approach to maximize therapeutic ratio. This risk–benefit analysis becomes even more important with the advent of molecular targeted agents that offer a viable alternative option for concurrent therapy.
The Emerging Role of Molecularly Targeted Agents
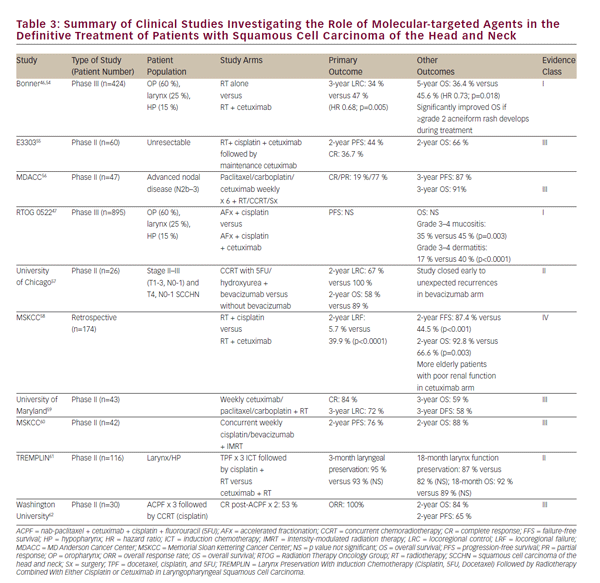
The toxicity of chemoradiation treatment, as well as a desire to further improve clinical outcomes, has fostered a burgeoning interest in the role of molecular-targeted agents for the treatment of SCCHN. EGFR is overexpressed or mutated in 80–90% of SCCHN tumors and this is associated with decreased response to radiotherapy and poorer clinical outcomes.43–45 In 2006, Bonner et al. reported on a landmark trial in patients with locally advanced SCCHN treated with either radiation alone or radiation concurrent with the EGFR antibody, cetuximab. This study demonstrated that concurrent cetuximab and radiation increased locoregional disease control and overall survival without exacerbating common toxicities of radiation.46 This study defined a role for concurrent cetuximab and radiation in patients who are not candidates for cisplatin.
Subsequent studies have sought to compare CCRT with concurrent cetuximab to that with cisplatin. Retrospective and early-phase studies have differed in this assessment and a randomized phase III comparison is underway in RTOG 1016. Notably, the RTOG 0522 trial did evaluate the potential added benefit of cetuximab to a cisplatin-based regimen of CCRT47. This study showed that when added to standard platinum based-CCRT, cetuximab did not further improve progression-free or overall survival. In the postoperative setting, RTOG 0920 will evaluate adjuvant radiotherapy with or without cetuximab in locally advanced resected SCCHN while the phase II/III RTOG 1216 trial will assess adjuvant CCRT with concurrent cisplatin versus docetaxel versus docetaxel and cetuximab for high-risk SCCHN.
Numerous early-phase clinical trials of additional molecular-targeted agents together with radiation for treatment of SCCHN have been conducted or are ongoing. A selection of these is summarized in i>Table 3. With the exception of studies on cetuximab, no other molecularly targeted agent has advanced to a point of influencing current practice outside of a clinical trial setting. While the prospect of molecularly targeted agents is promising, concurrent highdose cisplatin chemotherapy remains the preferred, level 1 recommendation of current National Comprehensive Care (NCCN) guidelines for CCRT.
Chemoradiation Therapy in Head and Neck Cancer—An Evolving Paradigm
The last 20 years have seen an emergence of chemoradiation as a standard of care in the definitive treatment of SCCHN. In appropriately selected patients the demonstrable survival benefit of this treatment outweighs the risks for additional toxicities. However, important questions remain regarding the optimal use of chemoradiation. A number of clinical trials have further explored the potential clinical utility of ICT in locally advanced SCCHN patients and while these studies have demonstrated improvement of ICT regimens at the expense of toxicity, none has conclusively demonstrated meaningful clinical improvement compared to cisplatin-based CCRT.
Future studies will no doubt continue to explore alternative chemotherapy regimens and dosing schedules as well as radiotherapy fractionation, dosing, and delivery methods in an effort to advance existing techniques. Moreover, with continued development of novel molecular-targeted chemotherapeutics, future studies will almost certainly define a burgeoning role for these agents in the treatment of SCCHN. Particularly intriguing may be the use of these agents in an induction setting prior to CCRT, where they may target micro-metastatic disease and decrease distant disease relapse without imposing toxicity-related limitations on CCRT completion rates. While use of CCRT is evolving, continued investigation into the appropriate role of surgery in SCCHN is also warranted. Advances in both robotic surgery and chemoradiation treatment approaches provide equipoise for such investigation. Current practice guidelines recommend adjuvant chemoradiation for postoperative patients with positive margins or ECE, while adjuvant radiation alone or consideration of chemoradiation is recommended for postoperative patients with a pT3 or pT4 primary, pN2 or pN3 nodal disease, level IV or V lymph node involvement, or with peri-neural or lympho-vascular invasion. Additional studies identifying preoperative radiographic and clinical predictors of high-risk status among patients with early-stage SCCHN will prove valuable in guiding the treatment of such patients who might be spared a surgical intervention with an up-front definitive chemoradiation treatment approach.
The recent recognition of human papilloma virus (HPV) infection as a cause and favorable prognostic factor in SCCHN originating from the oropharynx48 and potentially other sites is transforming the conception of SCCHN as a disease entity. In a retrospective analysis of RTOG 0129, 3-year overall survival for patients with tumors positive for HPV was 82.4% versus 57% for HPV-negative disease (p<0.001). Similarly, the independent prognostic value of p16-positivity as a surrogate for HPV status has been confirmed in retrospective analyses of TROG 0202 and DAHANCA 6 & 7.49,50 In light of this, future studies will necessarily be stratified by HPV status or tailored to a subset of HPV-positive or -negative patients, prompting a growing schism in treatment approaches. Already being raised is the prospect of investigating less-toxic treatment approaches for HPV-positive tumors and more aggressive definitive treatments for HPV-negative tumors in order to account for stark differences in their respective responses to current treatment modalities. RTOG 1016 is one such study specifically assessing HPV-positive patients for treatment de-intensification by use of concurrent cetuximab with radiation versus standard cisplatin-based CCRT. The interaction of specific treatment regimens and particular molecular-targeted agents with tumor HPV status will no doubt prove fertile ground for future research. It remains to be seen what impact the burgeoning HPV epidemic and current vaccination programs will have on clinical outcomes and treatment approaches in patients with SCCHN.







