Fungal laryngitis is an uncommon condition. The exact incidence is not well defined as very few cases have been reported in the literature. Candida is the most common pathogen involved, with Aspergillus species coming in second.1–3 Other rare causes of fungal laryngitis such as cryptococcosis, blastomycosis, and histoplasmosis have also been reported.4,5 This case report will focus on laryngeal aspergillosis, which can be classified into chronic non-invasive, allergic, and invasive forms.6 The disease process relies on two aspects: firstly, the direct clinical and pathological resemblance to other disorders of the larynx, specifically laryngeal carcinoma and premalignant lesions, which can often make the diagnosis challenging with a considerable impact on management. Secondly, identifying at risk patients is fundamental even in the absence of gross immunosuppression as this allows for earlier diagnosis and treatment, which is crucial for the invasive forms. We describe herein the case of a 66-year-old man, initially suspected to have a supraglottic laryngeal carcinoma with vocal cord paralysis that was finally diagnosed with an invasive epiglottic aspergillosis with cartilage destruction and abscess formation.
Case report
A 66-year-old man presented to the emergency department with a sore throat and low grade fever of three days duration associated with dysphagia and odynophagia. The patient was a heavy smoker and moderately consumed alcohol. He complained of progressive dysphonia over several months, which he attributed to his smoking history. He suffered from diabetes mellitus managed with oral metformin therapy.
Head and neck examination did not reveal any trismus or significant pathology of the oral cavity and oropharynx. No palpable neck masses or pathologic lymph nodes were identified. Therefore, a nasal fibroscopic exam was done and revealed the presence of a midline epiglottic ulcer extending to the left aryepiglottic fold and piriform sinus with consequent paralysis of the left vocal cord. The patient was suspected to have an ulcerative supraglottic laryngeal carcinoma with suppuration and possible abscess formation, and was admitted and administered intravenous (IV) clindamycin and dexamethasone. A chest X-ray was performed and was unremarkable. A neck computed tomography (CT) scan with IV contrast and neck magnetic resonance imaging (MRI) were carried out and suggestive of a tumoral process with necrosis and suppuration (see Figures 1 and 2). A few days later and after clinical improvement, microlaryngeal surgery for debridement and

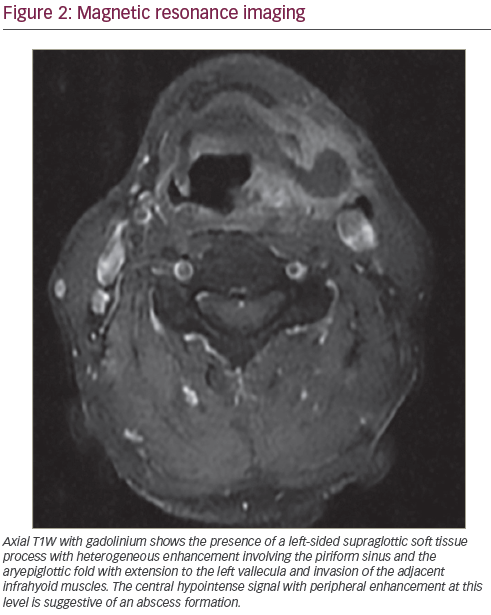
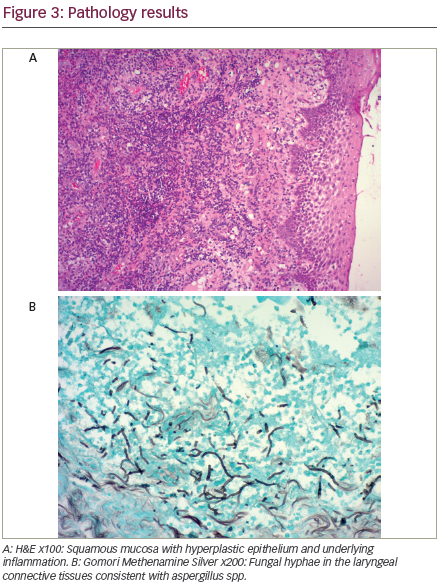
biopsy was performed and the patient was discharged on clindamycin to subsequently be managed for laryngeal cancer after the infection subsided. The pathology results indicated the presence of an invasive epiglottic aspergillosis with no evidence of malignancy and after worsening of symptoms on clindamycin the patient was readmitted and started on IV voriconazole (see Figure 3A and B). Stable glycemic control was achieved and HIV testing came back negative. A further microlaryngeal surgery with debridement was performed. The patient showed clinical improvement and was discharged on oral voriconazole therapy for three weeks.
Two months after treatment, the patient’s symptoms were completely resolved. Nasal fibroscopic exam failed to show any residual disease process in the epiglottis, aryepiglottic folds, or piriform sinuses with normal appearance and mobility of vocal cords bilaterally.
Discussion
Fungal laryngitis is an uncommon disorder. Few cases have been reported in the literature. Candida is the most common pathogen involved1–3 with Aspergillus species, specifically Aspergillus fumigatus, being the second most common. However other fungi have also been reported to be involved such as histoplasmosis and blastomycosis in endemic areas as well as Cryptococcus species.4,5
Fungal laryngitis usually affects individuals with predisposing conditions. These can be divided into two categories: systemic and local. The former involves any condition altering the host immune response to pathogens and this includes transient and permanent forms of immunosuppression (agranulocytosis, cell mediated immunodeficiency, systemic steroids, or other immunosuppressive drugs, etc.) as well as other disorders affecting immunity such as uncontrolled diabetes mellitus and nutritional deficiencies. The latter consists of factors altering the local mucosal barrier of the larynx and inducing microtrauma in the vocal cord ultrastructure. Smoking, the use of inhaled steroids in asthma patients, prior radiotherapy to the head and neck, prior intubation or laser surgery, and vocal abuse have all been described in patients with fungal laryngitis. Alteration of the local microflora of the larynx in subjects receiving multiple antibiotics or cytotoxic drugs have also been reported to be a predisposing factor for fungal colonization of the larynx.7,8 Recently, increasing numbers of cases involving women of childbearing age have led to the speculation that oral sex may play a role in the pathogenesis of fungal laryngitis.9
Alteration of the mucosal barrier with dysfunction of the local immunity of the upper aerodigestive tract appears to have a strong impact on the etiopathogenesis of fungal laryngitis, especially when the laryngeal involvement is isolated.7 This is important as cases of fungal laryngitis have been increasingly reported in patients without any history of gross immunosuppression. However, this association remains hypothetical because of the small number of cases reported in the literature. In fact, in as many as 50% of cases described in immunocompetent subjects, no significant contributing factor could be identified.9 Furthermore, it has been reported that fungi such as Aspergillus fumigatus can colonize true vocal cord cysts7,10 as well as other anatomic abnormalities of the larynx, such as laryngoceles among others.11
Laryngeal aspergillosis is a rare condition that was classically considered to affect exclusively immunocompromised patients. It is usually related to Aspergillus fumigatus, an ubiquitous, non-pathogenic, or weekly pathogenic organism that is commonly found in soil. As a result, laryngeal aspergillosis can often be regarded as an occupational hazard with farmers and carpenters being most commonly affected.7,12 From 1969–2015, around 50 cases of laryngeal aspergillosis have been reported in the literature of which 27 cases were identified in immunocompetent patients.9
The severity of the disease caused by Aspergillus species is variable and can be subdivided into three groups: chronic non-invasive, allergic, and invasive forms.6 These classifications can also be applied to Aspergillus infections of the larynx. The chronic non-invasive and allergic forms are usually indolent and present as a persistent non-healing laryngitis refractory to conservative therapy.1 On the other hand, the invasive form can be rapidly progressive and life-threatening and usually affects patients with a severely compromised immunity.
The laryngoscopic appearance of laryngeal aspergillosis is highly variable and can mimic other disease processes of the larynx, thus the diagnosis should rely on a high index of clinical suspicion and can be often challenging. The spectrum of the disease is wide and can range from simple congestive edema of the vocal cords in the noninvasive forms to white plaques mimicking leukoplakia and ulcerative or mass lesions nearly identical to neoplastic pathologies of the larynx.8 In the invasive forms, caseiting granulomas with abscess formation and sometimes diffuse sloughing and necrosis of the mucosa can be seen.13 Any part of the larynx can be involved. Besides laryngeal cancer, the differential diagnosis includes granulomatous disorders affecting the upper airway such as sarcoidosis, hematologic malignancies infiltrating the larynx, vasculitic disorders, and laryngeal tuberculosis among others.14
Invasive laryngeal aspergillosis is even more uncommon and is usually seen exclusively in the immunocompromised. The presence of an associated sinus, pulmonary and/or oropharyngeal involvement is usually the norm and the infection is often rapidly progressive with poor prognosis. A review by Ohashi et al. published in 2015, stated that only nine cases of invasive laryngeal aspergillosis have been published in the literature.14 The prognosis was dismal with a mortality rate exceeding 50%. An additional case of invasive laryngeal aspergillosis was recently published by Law and Reyes incriminating the use of inhaled antibiotics as a potential culprit for the development of this unusual disease.15
The diagnosis of fungal laryngitis and specifically aspergillosis can be difficult. In the noninvasive forms, the combination of suspicious laryngoscopic appearance along with a high index of clinical probability is often sufficient to start an empiric antifungal therapy with histopathologic confirmation required in refractory cases and in cases with a high suspicion for laryngeal malignancy.1 In the invasive forms, initiation of antifungal therapy as soon as possible is a must considering the highly aggressive nature of the disease.6 Otherwise, the diagnosis will be based primarily on histopathologic examination as fungal cultures are often negative.14 The use of β-D-glucan and Aspergillus antigen levels as a marker for invasive aspergillosis exhibits a promising high sensitivity16 but is not widely available and was not done in our case.
The histopathologic diagnosis of fungal laryngitis may be challenging. The inflammatory process accompanying fungal laryngitis may be associated with epithelial hyperplasia, hyperkeratosis, and acanthosis along with neutrophilic infiltration of the upper epithelial layers and scarring of the submucosa.1 These changes may be so pronounced in some cases that they are collectively termed as pseudoepitheliomatous hyperplasia, and can be mistaken for a malignant or premalignant process involving the larynx. The presence of neutrophils in the upper layers of the hyperplastic epithelium, the intensity of the inflammatory component, and the lack of definite malignant features should always trigger a search for fungal microorganisms.8 Special stains such as Periodic Acid-Schiff (PAS) and Gomori Methenamine Silver (GMS) can be used to highlight the fungal microorganisms when they are not readily identified. Alternatively, fungi may colonize a pathologic laryngeal ulcer related to an underlying malignant process. In rare cases, an intense inflammatory reaction with fungal hyphae and necrosis may mask the presence of an underlying malignancy,13 thus highlighting the importance of following the patient and monitoring his response to therapy.
Starting early empiric antifungal therapy when there is any suspicion of invasive laryngeal aspergillosis is crucial as the disease can be rapidly progressive and life threatening.6 Voriconazole has become the drug of choice for invasive aspergillus infection.6 The optimal duration of treatment for this entity is not clear due to the rarity of clinical studies. Duration should be guided by clinical response and reversal of the immunosuppressive state, the most important prognostic factor.6 Surgical debridement is often needed and will hasten recovery and in extreme cases as described by Richerdson et al, a total laryngectomy may be required.17 Amphotericin B, an echinocandin such as caspofungin, posaconazole, and itraconazole can all be used as salvage therapy for refractory cases.6 Noninvasive forms can be managed in an outpatient setting with oral itraconazole 100 mg twice daily for 3 weeks or even with topical nystatin (pastille or oral suspension) especially in immunocompetent patients.1
Conclusion
A wide spectrum of disorders of the larynx should be kept in mind in any patient presenting with a laryngeal ulcerative or granulomatous lesion. Although laryngeal malignancies would be the first to come to mind, other disease processes, fungal infections in particular, should also be considered. The initial presentation is often misleading as the laryngoscopic appearance can be highly suspicious for a malignancy including the presence of an associated vocal cord paralysis; furthermore, laryngeal involvement can be the only manifestation of invasive aspergillosis in at risk patients making the diagnosis even more challenging. Although appropriate antifungal therapy is to be started empirically when invasive fungal laryngitis is suspected, the presentation of the disease can be atypical and non-specific that targeted therapy will often be delayed until a pathologic diagnosis is obtained. Overall, the prognosis of any invasive aspergillus infection is usually dismal and will depend primarily on the immune status of the patient.







