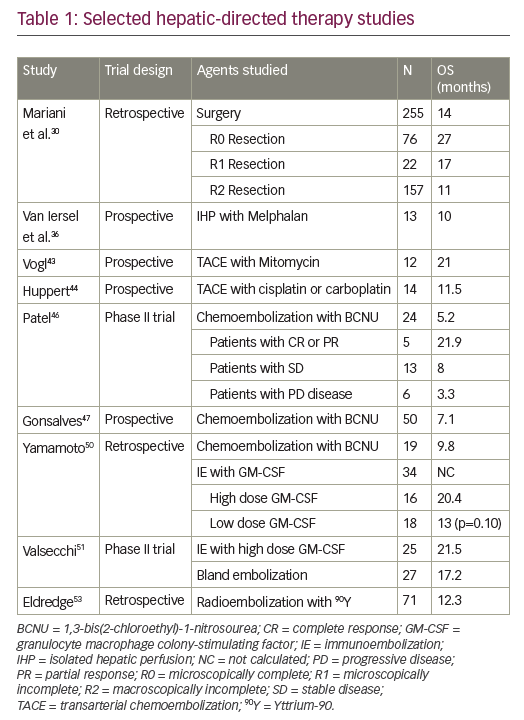
In the initial phase of treatment in acute myeloid leukaemia (AML), patients receive chemotherapy with the intent to eradicate malignant blast cells from bone marrow and other tissues. Induction therapy usually induces the disappearance of microscopically detectable malignant cells (complete remission [CR]), and the ensuing consolidation therapy aims at preventing the subsequent recurrence of leukaemia (relapse). The refinement of induction and consolidation protocols has drastically improved AML prognosis over the past four decades,1 but the occurrence of leukaemic relapse (typically within the first year after CR) is a major reason why the overall mortality of those diagnosed with AML remains worryingly high.
There are few therapeutic options available to prevent leukaemic recurrence. Patients may be subjected to allogeneic bone marrow transplantation (allo-BMT) from human leukocyte antigen (HLA)- matched siblings or unrelated donors, which efficiently reduces the relapse risk.1 Fewer than 30% of AML patients are eligible for allo- BMT, and the standard of care after the completion of chemotherapy is observation for most patients. The prevention of relapse, which occurs in 60–70% of all non-transplanted AML patients in CR,1 is a major challenge in AML. This article discusses the conceptual basis for immunotherapy with interleukin-2 (IL-2) in AML and reviews the results of clinical trials using IL-2 for relapse prevention. We also outline the rationale for combination immunotherapy with histamine dihydrochloride (HDC) and IL-2 as a post-consolidation remission maintenance strategy in AML.
Post-consolidation Immunotherapy with Interleukin-2 in Acute Myeloid Leukaemia
Two subsets of lymphocytes, T cells and natural-killer (NK) cells, are endowed with the capacity to recognise and destroy leukaemic cells. Immunotherapeutic regimens in AML have been developed to enhance the antileukaemic potential of these cytotoxic lymphocytes. The notion that T and NK cells participate in the surveillance of leukaemic cells in AML is supported by studies suggestive of a positive correlation between T/NK-cell function and a favourable disease outcome. This correlation is evident from studies in allo-transplanted patients, in whom T/NK-cells clearly mediate the graft-versus-leukaemia effect that forms the basis for relapse prevention.1,2 A similar correlation has also been found in non-transplanted patients.3–10 A therapy that improves antileukaemic functions of T and NK cells would have the potential to reduce the relapse risk in AML. The most frequently studied compound in this regard is the T- and NK-cell-activating cytokine IL-2.
IL-2, which is produced by T cells and is pivotal to several aspects of cell-mediated immunity, activates functions of T and NK cells such as antitumour cytotoxicity and production of cytokines (interferon-γ). In addition, it expands the population of T and NK cells by triggering cell-cycle proliferation. Beginning in the early 1990s, several single-arm trials were initiated using monotherapy with IL-2 to prevent relapse in the post-consolidation phase of AML. The results were considered encouraging. Subsequent controlled trials have yielded disappointing results. Five trials, the Blaise, CALGB 9720, CCG-2961, CALGB 19080 and ALFA 9801 trials, in a total of 905 patients have assessed the efficacy of IL-2 in prolonging leukaemia-free survival (freedom from relapse) versus the standard of care (no treatment) in AML patients in CR. None of these trials has shown significant beneficial effects of IL-2, given at monthly doses ranging between 25 and 91MIU,11,15 on any parameter of clinical efficacy. In the CALGB 19080 trial, a trend towards a reduced relapse rate (p>0.1) was observed in younger AML patients in their first CR (CR1), but other trials in younger CR1 patients are not supportive of the relapse-preventative efficacy of IL-2 monotherapy.14,15
Improvement of Interleukin-2 Efficiency by Histamine Dihydrochloride
Mechanisms of Immunosuppression in Acute Myeloid Leukaemia
The mechanisms responsible for the poor clinical efficacy of IL-2 in AML are not known in detail, but it seems reasonable to ascribe the lack of efficiency to a suppressed state of IL-2’s target lymphocytes in treated patients. In AML – and in several other forms of cancer – lymphocytes have been shown to be dysfunctional with, for example, a defective capacity to transduce activating signals and an increased propensity of undergoing apoptotic cell death.16,17 This phenomenon – often referred to as ‘cancer-related immunosuppression’ and a significant part of the larger entity of ‘cancer escape mechanisms’ – may serve to explain not only why malignancies arise and progress, but also why the efficacy of immunotherapies such as IL-2 are frequently insufficiently efficacious in human cancer. A treatment that counters cancer-related immunosuppression may unravel a therapeutic potential of IL-2.
Clinical Results of Combination Immunotherapy with Histamine Dihydrochloride and Interleukin-2
A recent phase III trial has demonstrated that treatment with histamine dihydrochloride (HDC, 0.5mg sq bid) in conjunction with low-dose IL-2 (14MIU/month sq bid) significantly prevented relapse in non-transplanted AML patients. The trial was performed in 10 countries with 320 participating CR patients. HDC/IL-2 was administered by the patients at home in a total of 10 three-week courses followed by three- to six-week rest periods for a total of 18 months. The therapy intended to coincide with the period of highest relapse risk after achieving CR. HDC/IL-2 therapy was acceptably well tolerated with no major impact on quality of life.17 The benefit of HDC/IL-2 appeared to be the result of the prevention of leukaemic recurrence in patients in first CR (CR1), in particular in those below 60 years of age (see Figures 1 and 2).
HDC/IL-2 therapy has been developed on the basis of pre-clinical studies suggesting that HDC improves the immuno-activating properties of IL-2.18 HDC promotes IL-2 activity by reducing or inhibiting suppressive signals from adjacent myeloid cells.
Pre-clinical Pharmacodynamics of Histamine Dihydrochloride
Studies in pre-clinical models imply that the principal action of HDC is to protect T cells and NK cells from inhibition and apoptosis induced by myeloid cells such as mononuclear or polymorphonuclear phagocytes. These myeloid cells confer inhibition of T- and NK-cell function and also trigger cell death by apoptosis in cytotoxic lymphocytes.19 HDC counters myeloid-cell-induced inhibition of T and NK cells by reducing or inhibiting the formation of reactive oxygen species (oxygen radicals) in several types of myeloid cells.20 The T and NK cells will remain viable, functional and reactive to IL-2 in the presence of myeloid cells.20 For example, the combination of HDC/IL-2, neither compound used alone, activates cytotoxic lymphocytes to efficiently lyse AML blasts in the presence of mononuclear phagocytes.21,22 In addition, HDC improves the IFN-γ- producing capacity of T and NK cells and efficiently improves the IL-2- induced appearance of activation antigens such as CD69 on the surface of these cells.23 Figures 2 and 3 depict these immunostimulatory properties of HDC, and Table 1 summarises the effects of HDC on T- and NK-cell function in vitro along with studies suggesting that the addition of HDC improves the antitumour activity of IL-2 in tumour-bearing rodents in vivo.
Clinical Pharmacodynamics of Histamine Dihydrochloride
During the course of the study using HDC/IL-2 in cancers other than AML, the effect of treatment with HDC/IL-2 on T- and NK-cell functions was monitored and compared with that of IL-2 monotherapy. In melanoma, patients receiving HDC/IL-2 showed improved induction of IFN-γ in several types of T cell,24 and in renal cell carcinoma, treatment with HDC/IL-2 was associated with improved functional status of cytotoxic lymphocytes in peripheral blood and at the site of malignant tumour growth.25
A tentative conclusion from these clinical pharmacodynamic studies is that treatment of cancer patients with HDC/IL-2 improves T- and NK-cell functions more efficiently than IL-2 alone, as predicted in pre-clinical models. These findings are considered relevant to the action of HDC and IL-2 in AML, since the studies demonstrate that HDC improves the efficacy of IL-2 on lymphocyte subsets that have been ascribed a role in the maintenance of leukaemia-free survival in AML. To clarify whether HDC improves the immune activation by IL-2 in AML patients, comparisons of the immunostimulatory properties of HDC/IL-2 versus IL-2 were performed during an initial phase II trial.21 Blood samples were drawn during an initial cycle of IL-2 monotherapy, and the level of accumulation of NK cells and activated T cells was compared with that achieved during a subsequent cycle with HDC/IL-2. The results showed a non-significant trend towards improved NK-cell expansion during the cycle with HDC/IL-2, along with a significantly more pronounced accumulation of activated T-cells (p=0.003; n=7) compared with IL-2 monotherapy. Although obtained in a small number of patients, these findings support that the HDC-induced potentiation of the immunostimulatory efficacy of IL-2 observed in other histotypes of cancer also occurs in AML.
Conclusions
Despite an impressive pre-clinical rationale, monotherapy with the T- and NK-cell-activating cytokine IL-2 has so far yielded disappointing results in clinical trials aimed at reducing the high frequency of relapse in patients with AML. The use of HDC in conjunction with IL-2 for relapse prevention in AML was founded on pre-clinical studies demonstrating that HDC improves the T- and NK-cell-activating properties of IL-2, specifically by countering suppressive signals from adjacent myeloid cells. Several functions of T and NK cells are improved by the addition of HDC to IL-2, and clinical pharmacodynamic studies support that a similar improvement of the immunostimulatory efficiency of IL-2 also occurs during treatment of patients with HDC added to IL-2. The results of a recent phase III trial demonstrate the superiority of HDC/IL-2 over the current standard of care in preventing relapse in AML patients. The treatment appears to be particularly efficacious in CR1 patients and in patients below 60 years of age. These findings imply that the countering of cancer-related immunosuppression may improve the clinical efficacy of IL-2 and may form the basis for the further development of immunotherapy as a relapse-preventative strategy in AML. ■