Cancers of the anal canal are relatively uncommon, and account for less than 1% of all new cancer diagnoses in the UK.1 However, the incidence of these cancers has increased over the past decade, primarily in females, in which they have more than doubled.1 Squamous cell anal carcinoma (SCAC) is the most common type of anal cancer, with adenocarcinomas, neuroendocrine carcinomas and melanomas being less common. Between 80% and 95% of these cancers are associated with the human papillomavirus (HPV).2–5 Another risk factor includes immunosuppression caused by, for example, HIV infection and organ transplantation.6
Most patients with SCAC present with localized or loco-regional disease, which is potentially curable. Definitive chemoradiotherapy with concurrent mitomycin C and fluorouracil is considered the standard approach in this setting and is associated with high cure rates in up to 60% of patients.7 In those with relapsed and/or metastatic SCAC, platinum-based chemotherapy is the mainstay of treatment, with carboplatin and paclitaxel considered the optimal regimen.8 Although associated with relatively high response rates (objective response rate [ORR] 59%), chemotherapy frequently provides short-lived responses. Prolonged, durable responses to platinum-based chemotherapy are uncommon, with most patients’ disease progressing within 9 months.8 In the second-line, chemorefractory setting, there are limited chemotherapy treatment options with established evidence in the treatment of metastatic SCAC, all with varying benefit and toxicities.
The use of immune checkpoint inhibitors (ICIs) is of particular interest in SCAC given the immunogenicity seen in these HPV-related cancers. The HPV oncoproteins (mostly E6 and E7) induce transformation from normal squamous epithelium to an invasive malignancy through inactivation of cell cycle proteins.9 These oncoproteins can induce immune escape through activation of programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) interaction, which downregulates tumour-infiltrating lymphocytes (TILs) and other immune regulatory cells.10,11 Blocking this pathway with ICIs can promote T-cell activation and enhance the host immune response, triggering tumour cell death. The rationale for the use of ICIs in the treatment of SCAC is based on the greater immunogenicity seen in these tumours relative to other, non-HPV-related cancer types. This article aims to review the current treatment landscape of SCAC, including the future use of ICI, with a focus on retifanlimab. We searched PubMed in August 2022. Search criteria and keywords used include “squamous cell anal carcinoma”, “immune-checkpoint” and “retifanlimab”. Only interventional studies were included in this review.
Retifanlimab as a programmed cell death protein 1 inhibitor
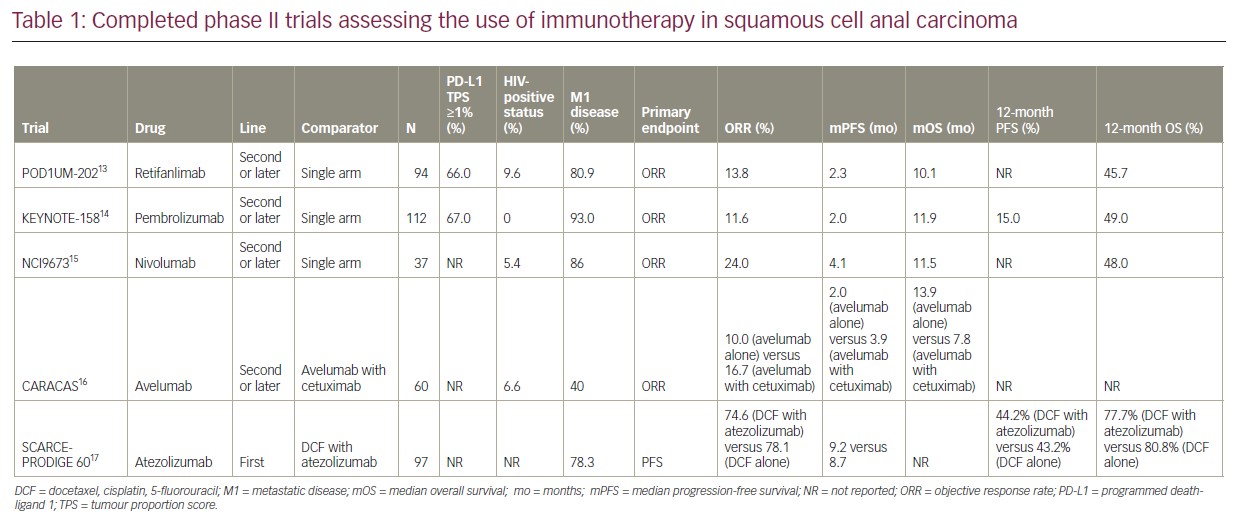
Retifanlimab is a humanized immunoglobulin G4 monoclonal antibody against PD-1 that blocks the interaction between PD-1 and PD-L1. It has activity against several solid tumours in the advanced setting, including gastric cancer.12 However, most of the evidence for its use is in the treatment of metastatic SCAC, with established safety and efficacy in the later-line setting. The POD1UM-202 trial was the first phase II study assessing the use of retifanlimab in the second- or later-line setting of locally recurrent or metastatic SCAC.13 Patients who had progressed on prior platinum-based chemotherapy were considered suitable for this study. The majority of patients enrolled had distant metastases (80.9%), 9.6.0% of patients had HIV infection, and 66% of patients had a PD-L1 tumour proportion score of ≥1%. Single-agent retifanlimab was intravenously administered at a dose of 500 mg every 4 weeks. Retifanlimab was associated with an impressive ORR of 13.8% (the study’s primary endpoint), including one complete response and a disease control rate of 48.9%. The median duration of response was 9.5 months. Responses were seen regardless of HIV infection, presence of liver metastases or age. The median overall survival (mOS) was 10.1 months, providing a signal of efficacy for the use of retifanlimab as a treatment option in those with chemorefractory SCAC. Although POD1UM-202 had no comparator arm, the mOS in those treated with retifanlimab is considered longer compared with single-agent chemotherapy in the second line. This study also aimed to investigate potential biomarkers of response to anti-PD-1 therapies; however, there was no association between tumour mutational burden (<10 mutations/Mb versus ≥10 mutations/Mb) and PD-L1 status on response, which is considered a predictive biomarker of response to ICI in other tumour types. In addition, there was no deterioration in HIV control in patients treated with retifanlimab, including no increase in the incidence of opportunistic infections. This is of particular importance given the risks of myelosuppression seen with cytotoxic chemotherapy. However, randomized data comparing chemotherapy with ICIs in this population is needed to assess efficacy and safety.
Importantly, retifanlimab was relatively well tolerated with treatment-related adverse events (TRAEs) of any grade seen in 58.0% of participants, with only 11.7% grade ≥3. The most common TRAEs were itch, fatigue, diarrhoea and nausea. Only 25.0% of patients experienced an immune-related adverse event (irAE), mostly related to endocrinopathy, skin involvement or pneumonitis (with pneumonitis leading to treatment discontinuation in one patient). The treatment discontinuation rate secondary to irAE was only 2.1%, considerably lower compared with other PD-1 inhibitors.
The POD1UM-202 study demonstrated the safety and efficacy of single-agent retifanlimab in patients with relapsed or metastatic SCAC, following progression on platinum-based chemotherapy. The efficacy of retifanlimab seems to be comparable to other PD(L)–1 inhibitors that have also been assessed in the treatment of advanced SCAC, as outlined in Table 1.13–17 Retifanlimab is currently being investigated in the first-line, phase III POD1UM-303/InterAACT 2 study (ClinicalTrials.gov Identifier: NCT04472429) of chemotherapy with or without retifanlimab in patients with relapsed or metastatic SCAC (as discussed below).18
Other anti-PD(L)-1 agents in squamous cell anal carcinoma
Pembrolizumab
Pembrolizumab is another PD-1 monoclonal antibody that has demonstrated clinical activity and safety in treating a range of advanced solid cancers, including SCAC. The KEYNOTE-028 phase Ib study assessed the use of pembrolizumab as monotherapy in patients with PD-L1-positive (i.e. ≥1%) advanced SCAC, following progression on at least one prior line of chemotherapy.19 Twenty-five patients were treated with single-agent pembrolizumab (10 mg/kg every 2 weeks). The primary endpoints were safety and ORR. Pembrolizumab was associated with an ORR of 17.0% and a disease control rate of 58.0%. TRAEs were seen in 64% of patients, most commonly diarrhoea and fatigue. There were no treatment-related deaths or discontinuation due to toxicities. Interestingly, one patient with non-squamous histology had evidence of stable disease, demonstrating possible activity even in less common subtypes of anal cancer. However, larger clinical trials are needed to validate this finding.
The KEYNOTE-158 phase II study further assessed the efficacy of pembrolizumab in a larger cohort of patients with locally advanced or metastatic SCAC in the second- or later-line setting.14 Unlike in the KEYNOTE-028 study, this study did not exclude patients who were <1% PD-L1-positive. Of the 112 patients enrolled and treated, 67.0% had PD-L1 positivity (≥1%), with 93.0% had metastatic disease. However, this study excluded patients with HIV infection, which were included in the POD1UM-202 study. Pembrolizumab was given at a dose of 200 mg every 3 weeks for up to 2 years. The ORR was 11.6%; however,ORR was slightly higher in those with PD-L1-positive tumours compared with PD-L1-negative tumours (15.0% versus 3.0%). The mOS was 11.9 months in all enrolled patients. Although 61.0% of patients had at least one TRAE, only 18.0% were considered grade 3–4. The KEYNOTE-158 study demonstrated efficacy and safety for the use of pembrolizumab as monotherapy in previously treated SCAC, regardless of PD-L1 expression.
Nivolumab
Another PD-1 inhibitor, nivolumab, has also demonstrated safety and efficacy in the treatment of SCAC. The NCI9673 phase II single-arm study assessed the use of nivolumab in patients with treatment-refractory, advanced SCAC.15 In an unselected PD-L1 population, 37 patients were treated with nivolumab at a dose of 3 mg/kg every 2 weeks. The ORR (primary endpoint) was 24.0%, with a complete response rate of 5.0%. One of the two patients who were HIV-positive that were included in this study had a partial radiographic response to nivolumab. Median progression-free survival (mPFS) was 4.1 months and mOS was 11.5 months. This is comparable to the survival data seen with pembrolizumab and retifanlimab in a similar cohort of patients. Unlike in the POD1UM-202 study, PD-L1 expression correlated with response rate, with responders having higher levels of PD-L1 expression compared with non-responders. However, this post hoc analysis only included a small sample size and further validation studies are needed to fully assess the validity of PD-L1 as a predictive biomarker of response to ICI in SCAC. In addition, larger studies assessing the use of nivolumab (and other PD(L)–1 inhibitors) in those with active HIV infection are needed to confirm the findings of the NCI9673 and POD1UM-202 studies.
Avelumab
Avelumab is an anti-PD-L1 agent that has efficacy and safety in patients with SCAC. The CARACAS study was a non-comparative, randomized phase II trial assessing the use of avelumab with or without cetuximab (anti-epidermal growth factor receptor [EGFR] monoclonal antibody) in the second- or later-line treatment of advanced SCAC.16 HPV-associated SCAC is characterized by high expression of EGFR, and combining ICI with anti-EGFR antibodies may theoretically enhance responses in SCAC.20 Patients were randomized to either avelumab with cetuximab (n=30) or avelumab alone (n=30). ORR (primary endpoint) was 10.0% with avelumab alone, compared with 16.7% when combined with cetuximab. The mPFS was longer in the combination avelumab with cetuximab arm compared with the avelumab alone arm (3.9 months versus 2.0 months). However, this did not translate to prolonged survival, with a mOS of 13.9 months in the monotherapy arm compared with 7.8 months in the combination arm. This study, however, was not designed to compare the two arms, with the authors concluding that the small sample size in both arms was a contributing factor for the incongruent result. Treatment with cetuximab and avelumab did slightly increase the rate of grade 3–4 TRAEs. This combination is not currently considered standard of care in the second-line setting of advanced SCAC, and further larger studies are needed to provide support for the use of avelumab (with or without EGFR inhibition) to understand the impact on survival.
Atezolizumab
Atezolizumab is another PD-L1 inhibitor that has demonstrated efficacy and safety in multiple solid advanced cancers. The randomized phase II SCARCE-PRODIGE 60 trial was the first study assessing the use of immunotherapy in combination with chemotherapy in the first-line setting of SCAC.17 Patients with untreated, locally advanced or metastatic SCAC were randomly assigned to either chemotherapy alone (docetaxel, cisplatin and 5-fluorouracil [DCF]) or in combination with atezolizumab. This study did not include patients with HIV infection. There was no improvement in 12-month PFS (44.2% versus 43.2%) or 12-month OS (77.7% versus 80.8%) between chemotherapy in combination with atezolizumab versus chemotherapy alone, respectively. The ORR was also similar between the two groups, 74.6% in the combination arm and 78.1% in the chemotherapy alone arm. There were also higher rates of grade 3–4 toxicities in the atezolizumab arm (59.0% versus 35.0%). This may be due to the concurrent use of immunotherapy and DCF chemotherapy, which may be mitigated with sequential treatment. This study did not show a benefit for atezolizumab in combination with chemotherapy in the first-line setting of advanced SCAC in a biomarker unselected population. However, it should be noted that there were baseline differences between the two arms, with those in the combination arm having worse prognostic features (i.e. bulkier disease and poorer performance status), which may account for the lack of survival benefit seen with this combination. Further first-line studies assessing the use of chemotherapy in combination with ICI in SCAC are ongoing (see Table 2).12,18,21–26
Ongoing trials
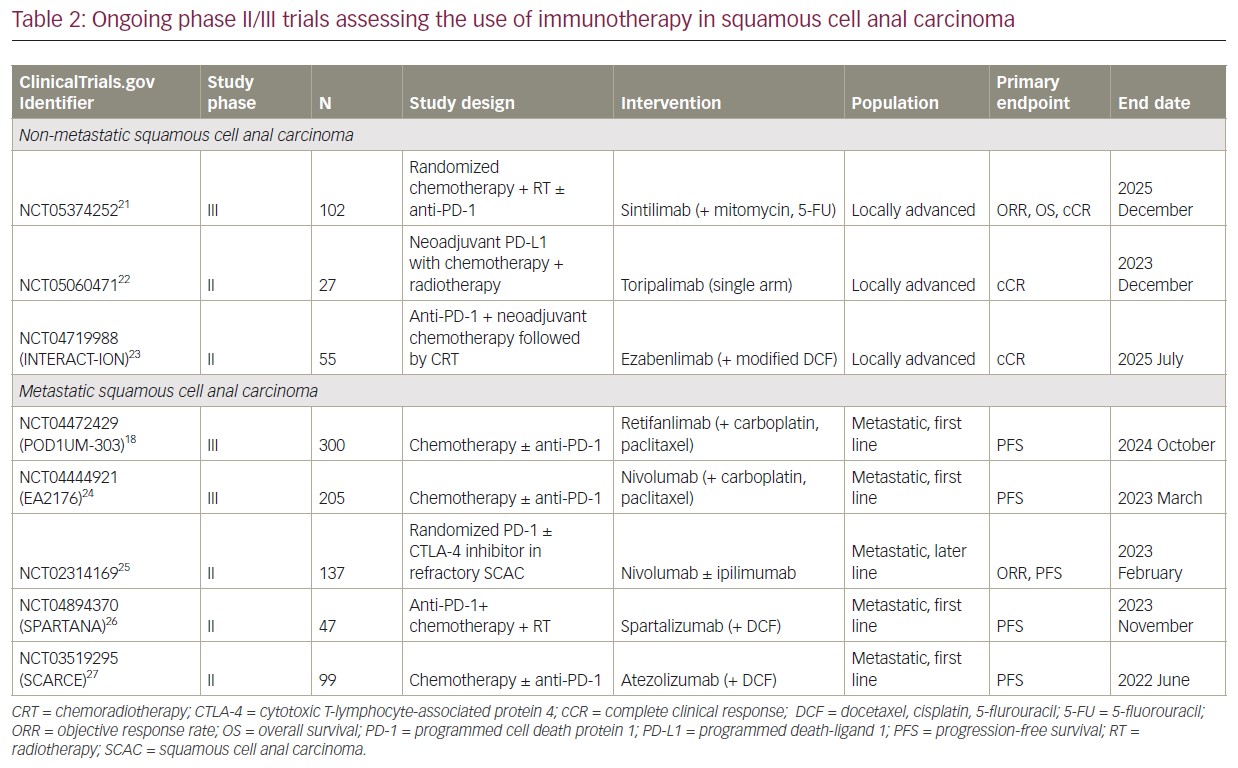
Multiple ICIs have demonstrated efficacy and safety in patients with unresectable locally advanced and metastatic SCAC, mostly in the second- or later-line setting. However, there is growing interest in their use earlier in the treatment paradigm in the first-line setting, in combination with cytotoxic chemotherapy and other agents.
The SCARCE-PRODIGE 60 trial, as outlined above, is the only trial to report on the use of chemotherapy in combination with ICI in the first-line setting of advanced SCAC. Although this phase II study did not meet its primary endpoint, other studies are assessing the efficacy of chemotherapy in combination with ICI in the first-line setting. The POD1UM-303/InterAACT2 study is a phase III randomized, placebo-controlled trial of chemotherapy alone (carboplatin and paclitaxel) or in combination with retifanlimab in the first-line setting of inoperable locally recurrent or metastatic SCAC in a PD-L1 unselected population.18 This study allows crossover from placebo to retifanlimab in those who progress in the chemotherapy alone arm. This study also includes patients with adequately treated HIV infection. Another randomized, phase III trial of chemotherapy alone (carboplatin and paclitaxel) or in combination with nivolumab in the first-line setting of locally recurrent or metastatic SCAC is ongoing in the EA2176 trial (ClinicalTrials.gov Identifier: NCT04444921).24
Studies in other tumour types have shown promise when combining immunotherapy and radiotherapy. The SPARTANA trial (ClinicalTrials.gov Identifier: NCT04894370) is assessing the use of spartalizumab (a PD-1 inhibitor) in combination with radiotherapy (single 8 Gy dose) and chemotherapy (DCF) in patients with advanced SCAC.26 This phase II study is based on the biological rationale that radiotherapy-induced DNA damage can promote tumour-specific neoantigen presentation and enhance TIL-mediated cytotoxic tumour cell death.
Given the efficacy of single-agent PD-1 inhibition in chemorefractory SCAC as outlined above, the use of PD-1 inhibitor in combination with cytotoxic T-lymphocyte-associated protein 4 inhibitors, such as ipilimumab, is of particular interest to enhance the immune-mediated response. A phase II trial of nivolumab with or without ipilimumab in treatment-refractory metastatic SCAC is assessing this combination as a chemotherapy-free regimen (ClinicalTrials.gov Identifier: NCT02314169).25
There is also growing interest in using ICI in early-stage disease given its efficacy in metastatic SCAC. The current treatment paradigm in this setting is concurrent chemoradiotherapy, and the use of ICI may enhance an antitumour immune effect when combined with both chemotherapy and radiotherapy. The INTERACT-ION trial is a phase II study of ezabenlimab (a PD-1 inhibitor) with chemotherapy (modified DCF), followed by concurrent chemoradiotherapy with capecitabine and mitomycin C (ClinicalTrials.gov Identifier: NCT04719988).23 Other clinical trials assessing chemoradiotherapy combined with ICI are ongoing (ClinicalTrials.gov Identifier: NCT05060471; ClinicalTrials.gov Identifier: NCT05374252).21,22
Conclusion
ICIs have shown promise as an effective and safe treatment in patients with advanced SCAC, following progression on first-line chemotherapy. The PD-1 inhibitor retifanlimab is associated with an encouraging response rate (13.8%) regardless of PD-L1 expression or HIV infection, with prolonged survival seen with single-agent retifanlimab in a chemorefractory setting. The efficacy of retifanlimab is comparable to other ICIs studied in this setting, including pembrolizumab and nivolumab. Retifanlimab also appears to be safe in patients with HIV infection, without higher rates of toxicity or loss of control of HIV infection, and can be considered the optimal therapy in this population. The toxicity profile of retifanlimab is similar to that seen with other ICIs, with no new safety signals seen in the POD1UM-202 trial.
As seen in the SCARCE-PRODIGE 60 trial, the use of ICI in combination with chemotherapy in the first-line setting of SCAC has been studied previously without improvement in efficacy and with higher rates of toxicity. However, further studies assessing this are currently ongoing. Chemotherapy in combination with ICI has shown promise in the treatment of other solid tumours, including metastatic lung cancer and gastric cancer. Chemotherapy induction of cellular death and presentation of neo-antigens to promote TILs and promoting cellular death provides the rationale for the ongoing phase III POD1UM-303 trial.
An important factor in the treatment of SCAC is identifying patients who will likely respond to ICIs. There is a paucity of data to support that PD-L1 status can be used as a biomarker of response to ICI in SCAC. Further validation of the use of PD-L1 status as a biomarker is the subject of interest in multiple ongoing clinical trials, including in the POD1UM-303 trial, to provide a personalized approach in the use of ICI to treat advanced SCAC.