Angiogenesis is defined as the process by which new blood vessels are formed, and research investigating how this physiological process is regulated has exploded in recent years. Targeting tumor angiogenesis has now become a well-validated approach for treating cancer, and first-generation angiogenesis inhibitors have been approved for clinical use since 2004. The development of effective angiogenesis inhibitors remains a high priority within the pharmaceutical community and continues to be a highly active avenue of new drug discovery.
The therapeutic inhibition of angiogenesis is clinically feasible since in normal physiology this process is required mainly during periods of rapid tissue expansion, such as development and childhood growth. In adults, angiogenesis remains tightly inhibited and is activated only at specific instances, such as wound healing or cycling of the female reproductive system. Inappropriate activation of angiogenesis is associated with a variety of pathologies including glaucoma, arthritis, and cancer. Since even cancer cells are dependent on the delivery of oxygen and nutrients, the expansion of a cancerous mass is also dependent on establishing effective vascularization. There is increasing evidence that the inhibition of angiogenesis is also a major mechanism by which the natural tumor suppressor pathways of human cells function. By understanding such pathways it may be possible to develop more effective regimens to target pathological angiogenesis in the clinic.
Angiogenesis is regulated by a variety of factors that exert effects on endothelial cells, the cellular building blocks of blood vessels. Angiogenesis-promoting factors include proteins such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF). In addition, a wide variety of secreted factors are also able to inhibit angiogenesis. Such proteins include thrombospondin-1 (Tsp-1), endostatin, tumstatin, and many others. The resulting balance of pro- and antiangiogenic signals sets the angiogenic output of a particular tissue or a tumor. Small masses of tumor cells usually undergo a process called the angiogenic switch, in which the production of pro-angiogenic factors increases dramatically and results in rapid tumor growth. The molecular mechanisms that toggle the angiogenic switch to the ‘on’ position are still largely unknown, but studies over the past few years have begun to reveal the extent to which growth signals that promote cell division are also tightly coupled to angiogenesis. Many of the oncogenes that drive tumor formation also induce expression of proangiogenic factors. Conversely, the products of genes that negatively regulate growth (i.e. tumor suppressors) are also potent inhibitors of angiogenesis.1 Tumor suppressors that have been demonstrated to inhibit angiogenesis include von Hippel- Lindau (VHL), the retinoblastoma protein (pRB), pTEN, and, as discussed in this article, p53.
Tumor Angiogenesis and p53
The gene encoding the p53 tumor suppressor, TP53, is mutated in half of all human cancers, and the correlation between p53 mutation and the aggression of cancers has been observed in dozens of published reports describing many different tumor types.2,3 Histological studies of human tumors have provided considerable links between p53 and angiogenesis. Tumors that have mutated p53 tend to be significantly more vascularized as measured by microvessel density (MVD) than tumors that retain functional p53. This correlation has been observed in several types of human cancers, including prostate,4,5 colon,5,6 breast,7 and head and neck tumors.8 Collectively, these clinical observations suggest that p53 can inhibit tumor formation, at least in part, by serving as a brake on tumor angiogenesis.
Experimental evidence has also shown that normal p53 function is required to reverse the angiogenic switch in cancer cells.9 Similarly, the expression of p53 has been shown to induce tumor dormancy through inhibition of angiogenesis.10,11 Small asymptomatic tumors, called carcinomas in situ (CIS), are thought to be very common in human tissues. For example, almost all adult individuals carry CIS in the thyroid, although only 0.1% of the population ever develops clinically diagnosed malignancy of the thyroid.12,13 Angiogenesis is a critical limiting factor in the growth of tumors, and without adequate vascularization the size of a tumor is limited to a few millimeters in diameter.14 Therefore, a critical event that may drive small incipient CIS to become aggressive and highly vascularized tumors may be the loss of functional p53. What then are the molecular events that allow p53 to limit angiogenesis and, more importantly, can the knowledge of such pathways lead to rational design of novel antiangiogenic therapies for cancer?
Molecular Mechanisms of Angiogenesis Inhibition by p53
The TP53 gene evolved in multicellular organisms for the main purpose of preventing premature death from cancer, which explains its high incidence of mutation in tumors. The p53 protein is downstream of several signal transduction pathways that detect DNA damage due to ultraviolet or ionizing radiation, and acts to destroy or isolate damaged cells to prevent them from growing into life-threatening cancers.15 Kinases such as the ataxia telangiectasia mutated (ATM) that are involved in sensing DNA damage are able to phosphorylate p53 in response to genotoxic stress. In addition, p53 becomes activated when oncogenes are expressed and cause unscheduled cell proliferation. Under normal conditions, p53 is kept under tight negative regulation by being continuously targeting for degradation. Upon activation by phosphorylation, the half-life of the protein increases dramatically and p53 protein levels accumulate in the cell and mediate its many tumor suppression activities.15 Among the activities of p53 implicated in preventing tumor growth is inhibition of angiogenesis. The inhibition of angiogenesis by p53 has been shown to occur through three basic mechanisms: downregulation of the hypoxia-inducible system (HIF), transcriptional repression of proangiogenic genes, and transcriptional upregulation of angiostatic factors. Each of these mechanisms will be discussed in turn.
Downregulation of Hypoxia-inducible System
A general property of all mammalian cells is the ability to detect when they are in an environment with insufficient oxygen (hypoxia). In response to hypoxia, cells activate a transcriptional program that promotes the production of new blood vessels to supply the oxygen-starved region. The central component of the hypoxia response is HIF1α.16 HIF1α is a transcription factor regulated by oxygen levels in tissues. Under conditions of normal oxygen levels, cytoplasmic enzymes called prolyl hydroxylases (PHDs) modify HIF1α by adding a hydroxyl group to one of two conserved proline residues. The resulting hydroyproline moiety then acts as a binding site for the VHL tumor suppressor protein, which targets HIF1α for degradation via the proteasome system.
PHDs use molecular oxygen as a substrate, and therefore their activity is dependent on oxygen tension in the cell. As oxygen levels decrease, so too does PHD activity, resulting in increased stability of HIF1α. HIF1α acts as a sequence-specific transcription factor and binds a cognate DNA sequence in the promoters of many genes required for hypoxia adaptation. The best characterized of the HIF1α target genes is VEGF, a major regulator of angiogenesis and the target of most existing antiangiogenic drugs. HIF1α is a central regulator of angiogenesis and p53 has been shown to directly inhibit its activity. The p53 protein was demonstrated to directly bind HIF1α and target it for degradation, even under hypoxic conditions.17 Thus, by binding and inactivating HIF1α, p53 prevents tumor cells from responding to hypoxia and stimulating angiogenesis.
Transcriptional Repression of Proangiogenic Genes
p53 functions as a classic transcription factor by regulating the expression of hundreds of target genes that mediate its tumor-suppressive effects. Although p53 is best known to increase the expression of target genes, in some cases it has also been shown to repress genes that promote tumor growth.18 In particular, p53 has been shown to repress a group of genes that are potent proangiogenic factors. The genes encoding two major secreted factors that promote angiogenesis in tumors, VEGF and bFGF, were both shown to be transcriptionally repressed by p53.19,20 Interestingly, p53 seems to have evolved redundant mechanisms to limit the production of VEGF both by degrading HIF1α, as discussed above, and by direct transcriptional repression of the VEGF promoter.
The gene encoding cyclo-oxygenase-2 (COX-2) has also been shown to be repressed by p53.21 COX-2 converts arachadonic acid to prostaglandin H2, which in turn can be converted to a variety of proinflammatory molecules called prostanoids. In addition to their role in inflammation, prostanoids have been shown to mediate production of proangiogenic factors and stimulate tumor growth. As a result of their effects in promoting angiogenesis, COX-2 inhibitor compounds are being evaluated as potential cancer therapeutics.22 Through direct inhibition of the transcription of these proangiogenic molecules, p53 is able to effectively limit the signals that cause endothelial cell division and formation of blood vessels.
Transcriptional Activation of Genes Encoding Angiostatic Factors
As mentioned in the previous section, p53 is best known as a transcription factor that promotes the expression of genes that limit tumor growth. Such genes encode proteins that induce cell-cycle arrest, mediate DNA damage, and repair and initiate apoptosis. These effects are classified as cell-autonomous in that they act directly on the cells that express p53. In addition, p53 regulates many genes that exert non-cell-autonomous effects, meaning that the cells expressing p53 are stimulated to produce factors that act on other cells and potentially other cell types. An example of such an effect is when tumor cells with activated p53 induce the transcription of secreted factors that have antiangiogenic properties. Some of the most powerful endogenous angiogenesis inhibitors have been shown to be under the control of the p53 tumor suppressor. Most angiogenesis inhibitors are proteins that are secreted into the extracellular matrix (ECM) and directly act upon endothelial cells to inhibit cell division or induce cell death.
One of the first characterized examples of an angiogenesis inhibitor, Tsp- 1, was also one of the first proteins demonstrated to be a transcriptional target of p53.23 Tsp-1 is a large 450kDa glycoprotein that is secreted into the ECM. Tsp-1 has been shown to inhibit angiogenesis through several mechanisms, including binding to a cell surface receptor on endothelial cells called CD36.24 Despite the fact that Tsp-1 is one of the most potent endogenous angiogenesis inhibitors, exactly how this factor inhibits angiogenesis remains poorly understood. Like p53, the expression of Tsp-1 has been shown to be able to completely reverse the angiogenic switch and render tumors dormant.9
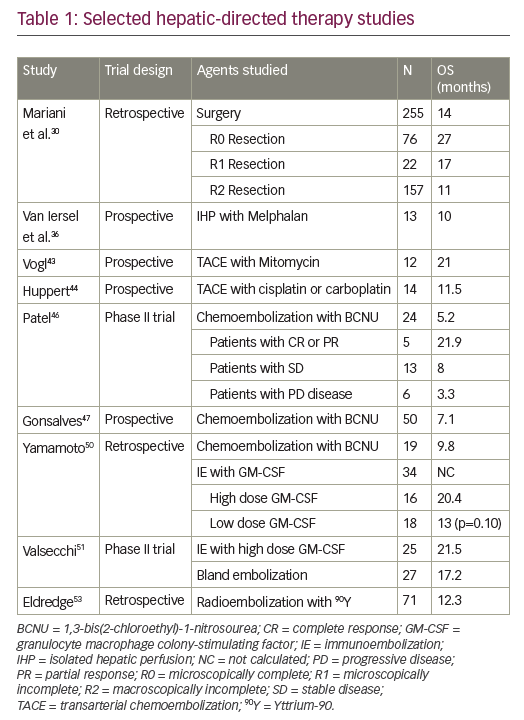
Many angiostatic factors are proteolytic fragments derived from components of the ECM. Among the many proteins that are secreted into the ECM are the collagens. Collagens are a family of about 40 proteins that have important structural properties in multicellular organisms. With respect to angiogenesis, collagen types 4 and 18 are critical components of a specialized ECM around blood vessels called the vascular basement membrane (VBM). Increasingly, such collagens have also been shown to be a significant source of signaling molecules that can affect angiogenesis. Six different types of collagen have been demonstrated to contain antiangiogenic C-terminal domains that can be released upon proteolytic processing (see Table 1). For example, endostatin is the best characterized collagen-derived antiangiogenic factor (CDAF) and is located at the C-terminus of collagen.18 Recent results from our own studies have indicated that p53 can stimulate the secretion of CDAFs by cancer cells into the tumor milieu, thereby limiting tumor vascularization.25
The collagen family of proteins is classified by the presence of the collagen repeats comprising the amino acid sequence Gly-X-Y, where X is often a proline residue and Y is a 4-hydroxyproline. A mature collagen molecule is formed when the collagen repeats of three separate collagen chains intertwined into a triple helix. Formation of this helical structure absolutely requires hydrogen bonding by the 4-hydroxyproline residues. Without these modified amino acids, the structure of the collagens would be unstable. For this reason, collagen production is dependent on the expression of the collagen prolyl hydroxylase enzymes in order to be formed. The most abundant collagen prolyl hydroxlase is P4HA1, which is responsible for producing the vast majority of collagen in connective tissues. Insufficient prolyl hydroxylase activity, which occurs when its essential cofactor vitamin C is lacking from the diet, causes scurvy, demonstrating the importance of this enzyme in collagen biosynthesis.
Recently, we were able to demonstrate that p53 upregulates the expression of another collagen, prolyl hydroxlase (P4HA2). Unlike P4HA1, P4HA2 is not ubiquitously expressed and is not likely to be involved in the formation of collagen in connective tissues. The overexpression of P4HA2 in lung cancer cells increased the production of collagen 4 and 18 and stimulated the release of the CDAFs tumstatin and endostatin.25 These data provided a genetic and biochemical link between p53 and the CDAFs. In addition to increasing expression of the prolyl hydroxylase enzyme required for collagen biosythesis, p53 has also been shown to upregulate expression of at least two different collagen genes. Two genome-wide screens identified both the COL18A1 gene26 (endostatin) and the COL4A1 gene (arresten)27 as transcriptional targets of p53. Thus, p53 is able to initiate an entire transcriptional program that stimulates cells to secrete CDAFs to inhibit tumor angiogenesis. Since p53 upregulates a collagen prolyl hydroxylase enzyme that is a rate-limiting step in collagen biosynthesis, this mechanism has the potential of increase production of any (or all) of the CDAF molecules listed in Table 1.
What Can p53 Regulation of Angiogenesis Teach Us About Designing Future Cancer Therapies?
Tumor-suppressor pathways such as p53 function in part by reducing the angiogenic potential of transformed cells. Limiting angiogenesis by p53 occurs through the dual action of inhibiting proangiogenic signals and increasing production of angiostatic factors. The major lesson that can be learned from p53 is that effectively shutting down angiogenic potential requires the execution of both of the above mechanisms.
Current cancer therapies targeting angiogenesis function exclusively by inhibiting proangiogenic factors. Indeed, existing angiostatic drugs such as bevacizumab, sorafenib, and sunitinib all function through the common mechanism of inhibiting signaling through the VEGF pathway—but what of the second mechanism of increasing angiostatic factors such as the CDAFs and Tsp-1? No significant progress has been made toward utilizing antiangiogenic factors such as Tsp-1 or CDAFs in the clinic despite years of pre-clinical data showing that these proteins are powerful antiangiogenic agents. One of the difficulties that has impeded discovery of drugs that function through antiangiogenic pathways is that the molecular mechanisms of these signals seem to be more complex than their proangiogenic counterparts. For example, Tsp-1 has been reported to interact with several different receptors, although much of the angiostatic activity is thought to be mediated through the CD36 receptor. The use of Tsp-1 as a therapeutic is also complicated by the fact that it is a very large protein that would be difficult to produce in sufficient quantities for treatment. This is not the case for the CDAFs, which are relatively small 16–20kDa peptides and can be adapted almost directly as drugs.
There is evidence that increasing production of CDAFs can have a significant effect on tumor growth. Studies in mice have shown that increasing levels of endostatin by only 1.6-fold can result in lower tumor growth.28 In this same study it was also shown that mice lacking the expression of the CDAFs endostatin or tumstatin result in two- to three-fold faster tumor growth. Unfortunately, when phase II clinical trials for endostatin were carried out for the treatment of advanced neuroendocrine tumors, no significant tumor regression was observed.29 The lack of response in this study may have been due to the type and late stage of the cancers under study, as well as the protocol of using endostatin as a monotherapy. Since this initial report, no further trials using CDAFs have been carried out at any sites in North America or Europe. However, several clinical trials are being carried out in China using a modified version of endostatin called Endostar. Results of trials using chemotherapy in combination with Endostar for treatment of lung cancer have been reported to show significantly greater tumor regression than patients receiving chemotherapy alone.30 Endostar has now been approved for the treatment of lung cancer in China and trials are in progress for the treatment of other cancer types.
We have observed the p53 tumor suppressor to stimulate the release of at least two different CDAFs, endostatin and tumstatin.25 It is likely that using cocktails of CDAFs may prove more effective. CDAFs function by binding to integrin molecules on endothelial cells, and each CDAF has slightly different properties.31 Since p53 can stimulate production of at least two different CDAFs, using such mixtures may be a more effective method of shutting down angiogenesis. Another strategy used by p53 that may prove clinically relevant is to utilize therapies that target proangiogenic signals such as VEGF in combination with CDAFs. Drugs that target VEGF such as bevacizumab have been approved for treatment of lung and colorectal cancer and are able to extend the life of patients by four to six months. Unfortunately, tumors eventually develop resistance to these medications and evolve other routes by which to promote angiogenesis. Preventing tumor vascularization may be analogous to fighting infectious diseases such as HIV. The control of angiogenesis in cancer may be achieved only through the application of well-designed cocktails of drugs targeting several aspects of angiogenesis biology. Tumor-suppressor proteins such as p53 are able to hold angiogenesis in check for extended periods of time, and studying such pathways may provide insights toward how tumor angiogenesis can be controlled in the clinic.■